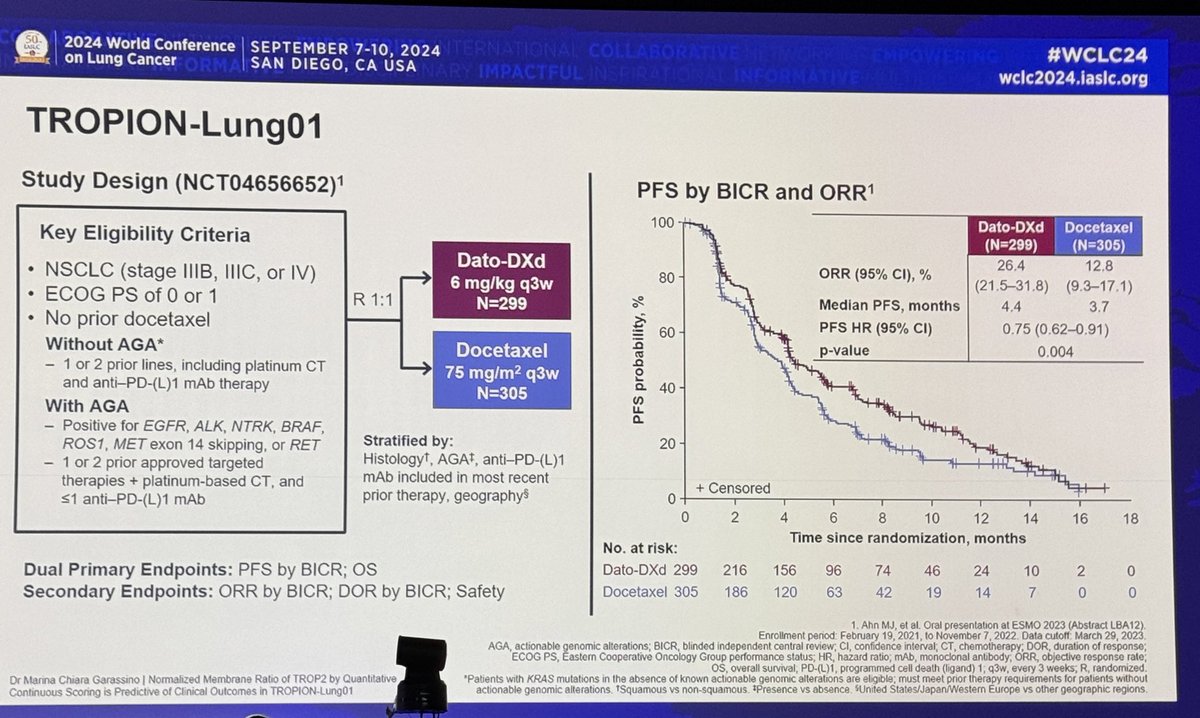
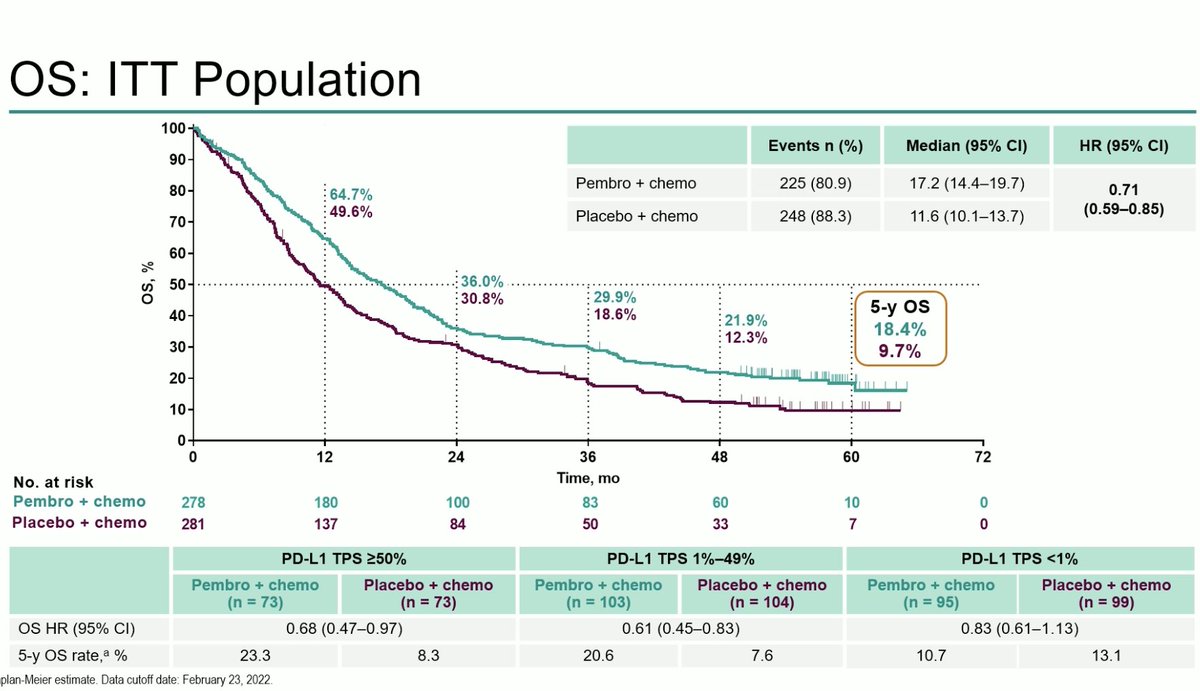
#ESMO20 Happy to share the first results of RAIN-701, a phase II study of #tarloxotinib in patients with NSCLC harboring an #EGFR exon 20 insertion or an activating #HER2 mutation and any solid tumor with an #NRG1 or ERBB gene fusion. #LCSM @OncoAlert 

#ESMO20 Tarlox is given as an inactive prodrug that embeds in the cell membrane. Only when it encounters hypoxia does it undergo single electron reduction to fragment to a cell permeable, pan-HER, irreversible TKI with subnanomolar potency at EGFR, HER2 and HER4. @OncoAlert #LCSM 

#ESMO20 Top right shows the IC50 of Tarloxotinib-E in human cell lines, bottom right corroborate with additional mutations in BAF3 models. Note the differential target inhibition for Tarlox-E with a lower IC50 for #HER2 than EGFR and the lowest IC50 for #NRG1 fusions. #LCSM 

#ESMO20 RAIN-701 enrolled three cohorts: (A) NSCLC EGFRex20, (B) NSCLC #HER2 mutation, and (C) any tumor with #NRG1, EGFR, or HER fusions. As of data cutoff June 12th, 23 patients enrolled, 83% with prior immunotherapy. #LCSM @OncoAlert @IASLC 

#ESMO20 Well tolerated and due to its design, low rate of wt EGFR-related toxicities. Most common G3 toxicity was QT prolongation but no arrhythmias. Only 1 patient discontinued therapy due to adverse event (infusion reaction). #LCSM @OncoAlert 

#ESMO20 Cohort B: tarloxotinib in #HER2 NSCLC: 8 pts with at least one scan, 2 confirmed PR and 2 with reduction of 24-27%. In those 4 patients, no diarrhea and no grade 3 rash. Design of the drug decouples efficacy with EGFR toxicity. Scans illustrating response on right. #LCSM 
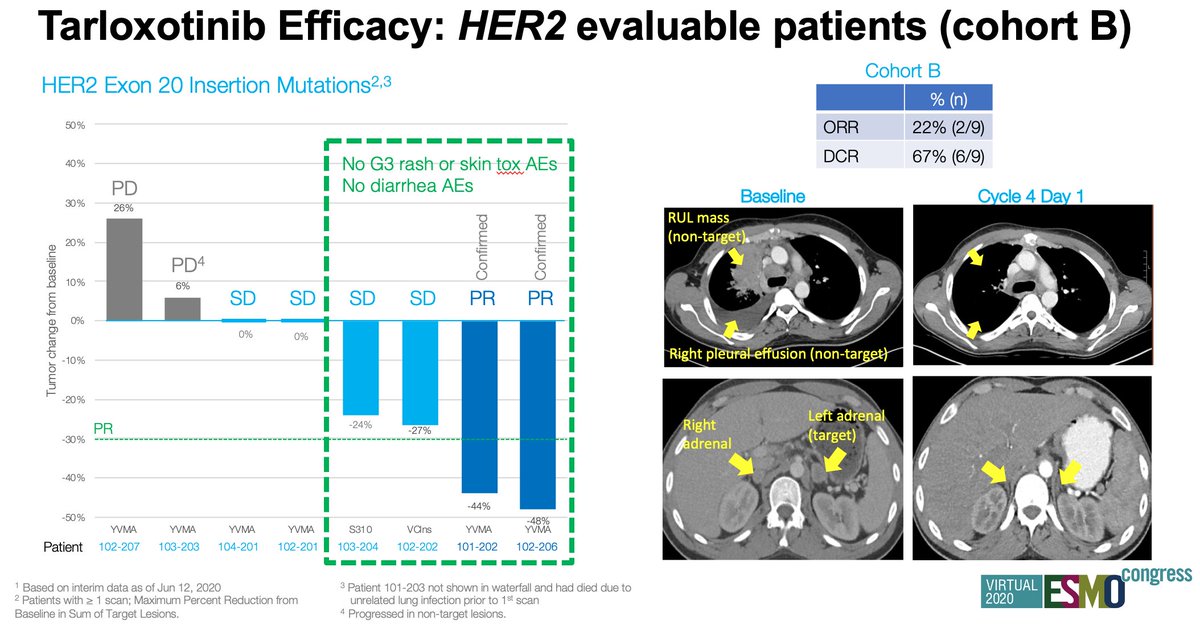
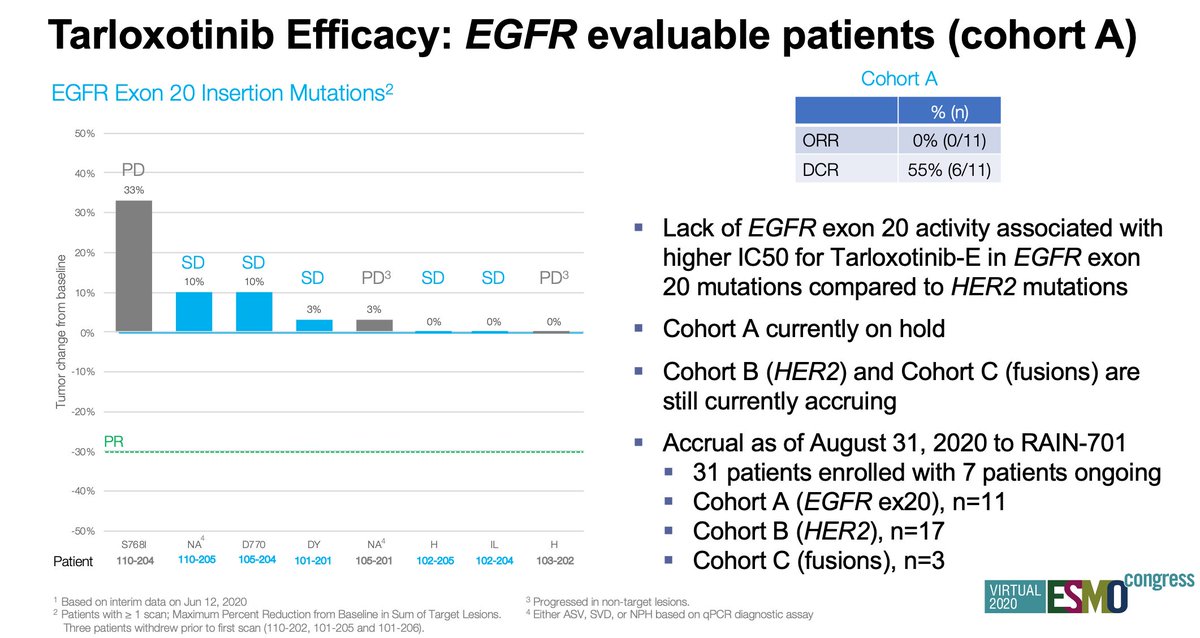
#ESMO20 In contrast, no responses seen in cohort A (EGFRex20). Correlates with preclinical data of higher IC50 for EGFR. This cohort is now on hold. Cohort B (HER2) and cohort C (NRG1 and EGFR/HER fusions) is enrolling. Accrual as of August 31 is 31 patients. #LCSM 
#ESMO20 The swimmers plot demonstrates duration of therapy for the first 23 patients. In addition to the confirmed responses observed, several patients achieved durable stable disease and with prolonged dosing, there was a lack of cumulative toxicity. #LCSM @OncoAlert 

#ESMO20 Overall, tarloxotinib demonstrated early efficacy in #HER2 NSCLC and its prodrug-hypoxia design decoupled efficacy with typical dose-limiting, on target, EGFR-related toxicity. RAIN-701 enrolling HER2 NSCLC and any tumor with #NRG1, EGFR, or HER gene fusions. @Rain_Thera 

#ESMO20 Appreciation to all patients as well as co-investigators @chulkimMD @ViolaZhu4 @CarolineMccoach @LungCancerDr @DocSacher @SchenkLab @Rain_Thera 

Felt compelled to share all of my slides here due to the technical difficulties with the website (I promise I turned it in on time!) @myESMO is working now to fix the technical issues but in the meantime, I encourage other authors to post their slides here! #ESMO20 

• • •
Missing some Tweet in this thread? You can try to
force a refresh