#ESMO20 Report of the IFCT-1601 IoNESCO study of neoadjuvant durvalumab monotherapy for resectable #NSCLC presented by @MarieWislez in the Proffered Paper session chaired by @LudaBazhenovaMD @FlorianaMorgill #LCSM @OncoAlert 

#ESMO20 Neoadjuvant checkpoint inhibitor monotherapy has shown efficacy in previous studies of resectable stage I-III NSCLC with major pathologic responses (MPR) seen in 17-45% of patients. @OncoAlert #LCSM 

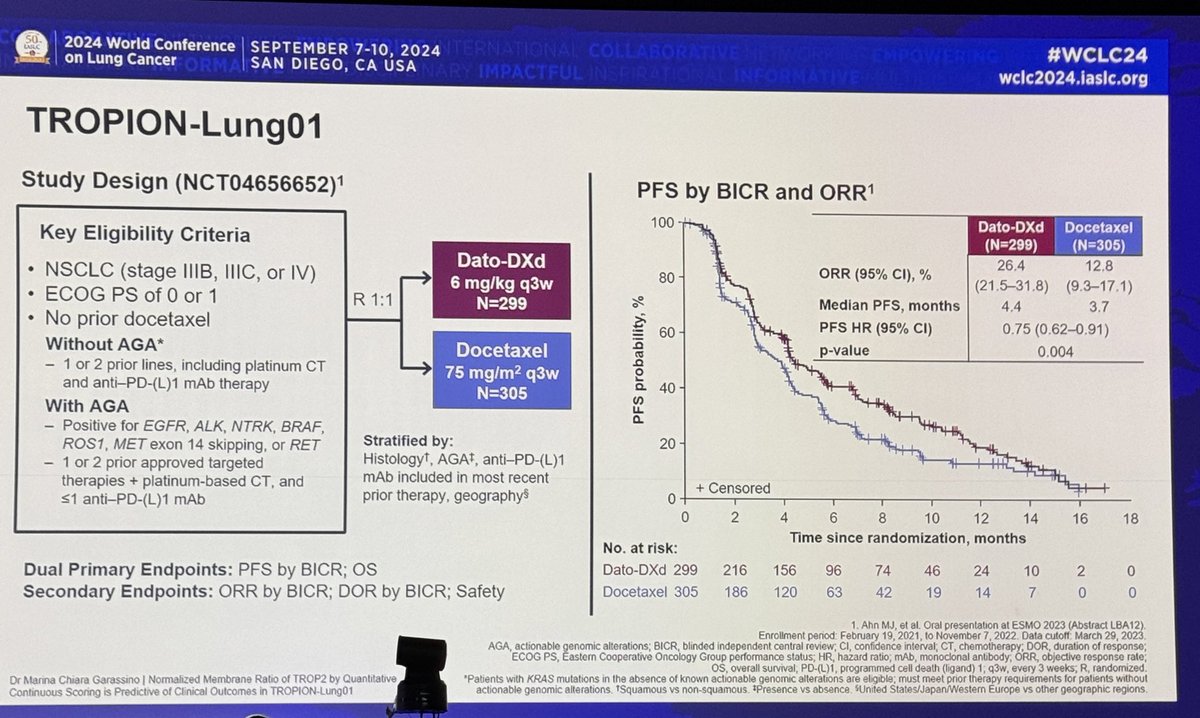
#ESMO20 Study design of IoNESCO IFCT-1601: included stage IB, II, IIIA (non-N2), treatment was three doses of durvalumab every 2 weeks followed by surgery within 2-14d of resection. Primary endpoint was feasibility of resection with 90d mortality as a secondary. #LCSM @OncoAlert 



#ESMO20 This study was terminated early for high 90 day post-operative mortality. Of the 46 patients treated, 43 went to surgery and of those, 5 deaths within 90 days #LCSM @OncoAlert 

#ESMO20 Demographics included here show primarily stage II (10% stage IB and 4% stage IIIA). 88% of patients received all 3 planned doses of neoadjuvant durvalumab. Relatively high number of pneumonectomies (20.5%) - will wait to see if that correlates to mortality. #LCSM 

#ESMO20 Surgery was primarily complete (R0, 89%). 1y OS rate 89% with 1y DFS rate 78.2% overall. #LCSM @OncoAlert 



#ESMO20 Important points here. RECIST RR only 8.7% but MPR rate 18.6%. The discordance between radiographic and pathologic response is consistent with prior reports from others (@FordePatrick) and important to remember when planning resection. #LCSM @OncoAlert 

#ESMO20 Outcomes better when MPR achieved with better OS and DFS (red curves) compared to those who did not achieve MPR (though a multivariate analysis would help here). #LCSM @OncoAlert 

#ESMO20 MPR did correlate with neovascularization, lymphocytes, cholesterol, fibrosis - but did not correlate with neutrophil infiltration or necrosis. #LCSM @OncoAlert 

#ESMO20 Turning to explaining the high 90d mortality, safety with durvalumab seemed quite good. No concerning signals here - no grade 3-5 AEs, no serious AEs, mostly mild grade 1 toxicities. #LCSM @OncoAlert 

#LCSM Here we see 4 of the fatal events (the flowsheet showed 5). All had R0 resections. One pneumonectomy who developed a fistula and one surgical complication. Sudden death and respiratory distress seen in the other 2. Not attributed to durvalumab. Possibly patient selection. 

#LCSM Overall, the study was stopped due to excess 90d mortality but felt related to patient comorbidities and not durvalumab or immunotherapy per se. MPR rate consistent with prior reports and correlation with MPR. Note discordance of RECIST with MPR for future studies. #LCSM 

• • •
Missing some Tweet in this thread? You can try to
force a refresh