#ESMO20 Eagerly awaited presentation by @peters_solange on consolidation nivolumab and ipilimumab (vs observation) for patients with LS-SCLC after chemoradiation: ETOP/IFCT 4-12 STIMULI trial #LCSM @OncoAlert @myESMO 

#ESMO20 While we have adopted IO for ES-SCLC, its role in LS-SCLC is not yet known, though outcomes in LS-SCLC are still poor. Nivo/ipi has activity in SCLC, but note that the consolidation/maintenance approach in ES-SCLC was not successful. #LCSM 



#ESMO20 Study design outlined here: enrolled at diagnosis or after 1 dose of chemotherapy to allow referrals (smart design). Standard concurrent CRT followed by 1:1 IO vs observation. Used nivo 1mg/kg and ipi 3mg/kg q3w x 4 cycles with maintenance nivolumab (not low dose ipi). 
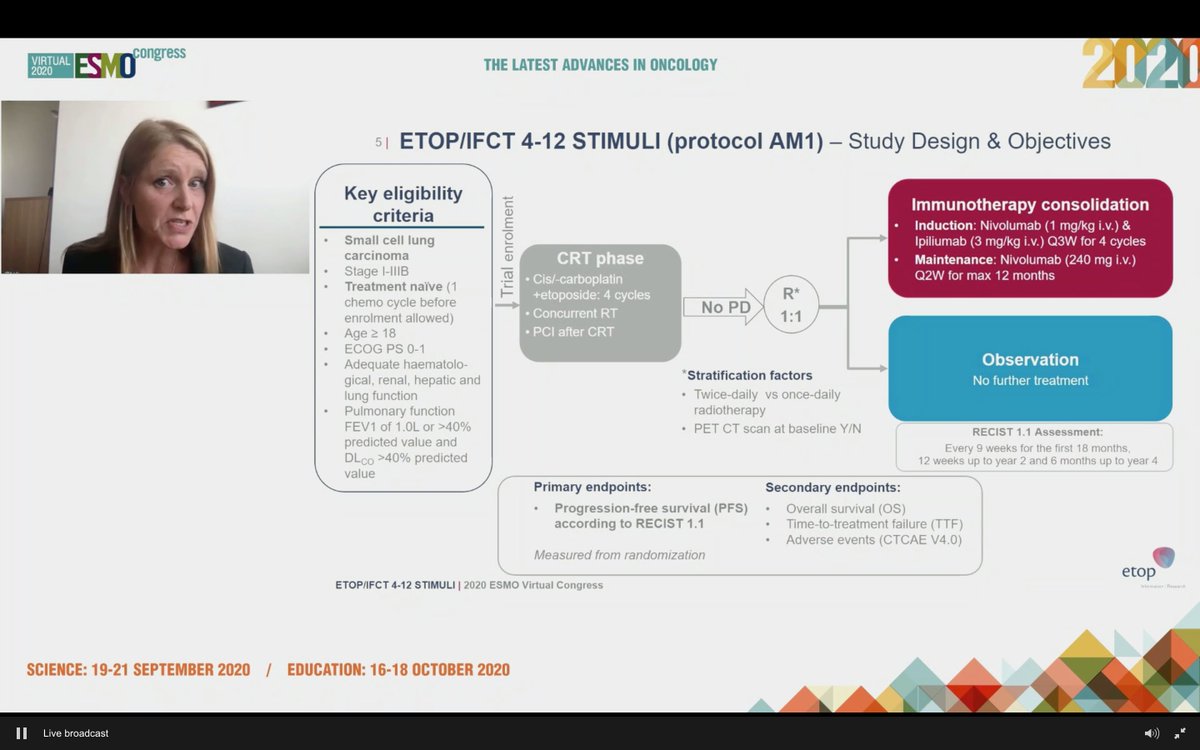
#ESMO20 Statistical design including explanation of premature closure to accrual which led to a pretty long period of accrual. Treatment failure in observation was due to progression whereas in nivo/ipi arm, it was toxicity. 





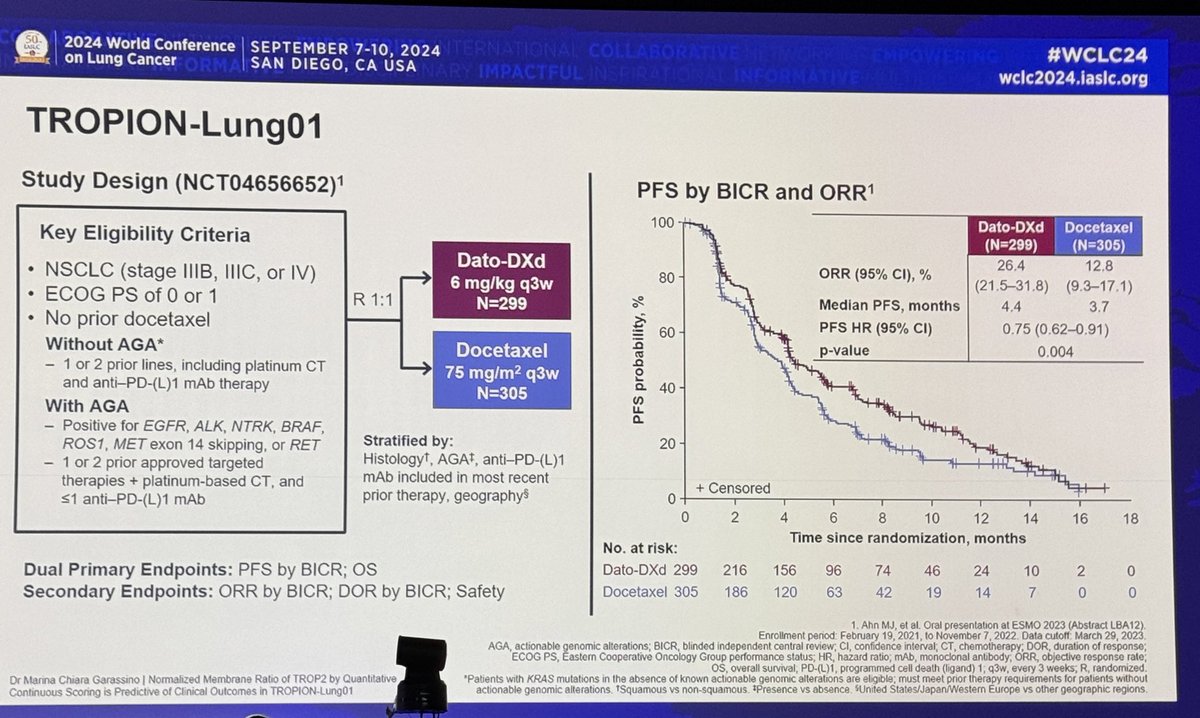
#ESMO20 Consolidation nivo/ipi after chemoradiation for LS-SCLC did NOT improve PFS. HR 1.02 with curves crossing multiple times. Median PFS for nivo/ipi was 10.7m versus 14.5m for observation. #LCSM @OncoAlert 

#ESMO20 Subgroup analysis without clear trends to identify groups that would benefit from nivo/ipi consolidation. Patterns of progression similar, mostly new lesions. #LCSM @OncoAlert 



#ESMO20 Consolidation nivo/ipi after concurrent chemoradiation for LS-SCLC did not improve survival with an OS HR 1.06. Interesting approach looking at piecewise hazard ratios and this may mature differently but as of now, no OS benefit. #LCSM 

#ESMO20 Disappointing OS results with nivo/ipi consolidation. Subgroup analyses here not very telling. More toxicity with nivo/ipi, naturally, vs observation, but nothing unexpected. #LCSM 







#ESMO20 Overall, the use of consolidation nivolumab and ipilimumab after concurrent chemoradiation for LS-SCLC did not improve PFS or OS. Longer follow up may inform late impact, though crossover at relapse may make that challenging. #LCSM @OncoAlert @myESMO 



#ESMO20 My immediate reaction is disappointing as there is a lot of rationale for IO post CRT in LS-SCLC. Is it the ipi - is the dose too high in a population with co-morbidities (worsened by CRT)? Parallels to CheckMate 451 (maint nivo/ipi in ES-SCLC did not improve OS)? #LCSM 

#ESMO20 This question of IO post CRT for LS-SCLC is still valid (better without CTLA4?). Consider referring to NRG-LU005 (chemoradiation +/- atezolizumab, NCT03811002) or ADRIATIC (chemoradiation followed by durva +/- treme, NCT03703297). #LCSM
clinicaltrials.gov/ct2/show/NCT03…
clinicaltrials.gov/ct2/show/NCT03…
• • •
Missing some Tweet in this thread? You can try to
force a refresh