#ESMO20 Discussion of the phase III CROWN trial results (planned interim analysis): lorlatinib vs crizotinib in treatment naive #ALK #NSCLC by @bensolomon1 - potentially practice changing data in an important subset of lung cancer. #LCSM @OncoAlert 

#ESMO20 #ALK fusion positive NSCLC is an important subset where we have very potent and active agents with PFS measured in years - but always room for improvement. #LCSM @OncoAlert 

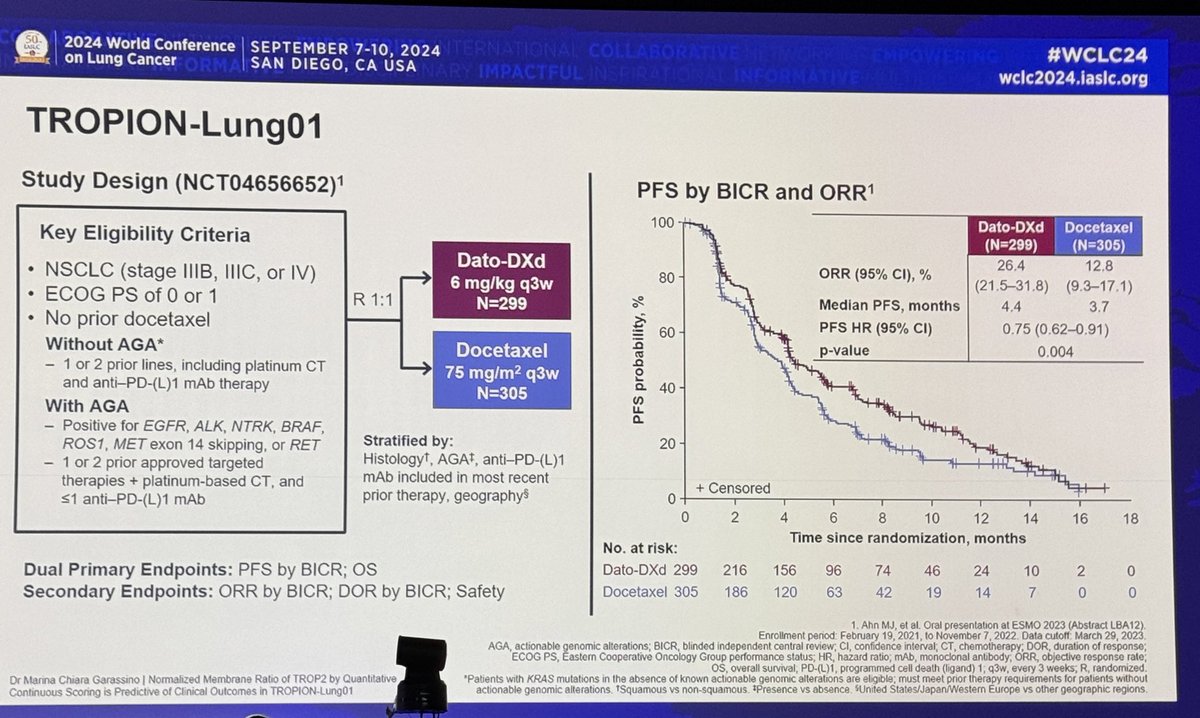
#ESMO20 Study design for CROWN shown here. Standard 1:1 randomization to lorlatinib 100mg qday vs crizotinib 250mg bid, stratified by Asian vs not and presence or absence of brain metastases. Primary endpoint was PFS by BICR. #LCSM @OncoAlert 





#ESMO20 Baseline characteristics show that 26% of patients had brain metastases (only 6% with prior radiation) and by central MRI review. #LCSM @OncoAlert 

Wow.
PFS by BICR HR 0.28 substantially better (across trials) than we saw with alectinib or brigatinib (0.47-0.49). 12 month PFS rate 78% with lorlatinib vs 39% with crizotinib. #LCSM #ESMO20 @OncoAlert
PFS by BICR HR 0.28 substantially better (across trials) than we saw with alectinib or brigatinib (0.47-0.49). 12 month PFS rate 78% with lorlatinib vs 39% with crizotinib. #LCSM #ESMO20 @OncoAlert

#ESMO20 Here we see investigator assessed PFS HR was 0.21 favoring lorlatinib over crizotinib as 1L therapy for #ALK NSCLC. #LCSM @OncoAlert 

#ESMO20 Benefit seen across all subgroups with a better HR in patients with brain metastases (HR 0.20) but still impressive in those without (0.32) but overall, benefit seen across the board. #LCSM @OncoAlert 

#ESMO20 ORR 76% with lorlatinib vs 58% with crizotinib. Both work quickly (median time to response 1.8m) but duration of response not reached for lorlatinib (11m for crizotinib). #LCSM @OncoAlert 

#ESMO20 Lorlatinib has greater CNS penetrance than other ALK TKIs and we see impressive CNS results in CROWN. Intracranial response rate in those with measurable disease was 82% with lorlatinib - 71% were complete responses! #LCSM @OncoAlert 

#ESMO20 The intracranial time to progression (BICR, MRI) not reached but the HR was 0.07 (!!!) and that lorlatinib line is about as flat as can be. #LCSM @OncoAlert 

#LCSM Very early look at OS - this will be critical to follow. As resistance mechanisms vary by agent used, we don't know much about resistance to first line lorlatinib and over time, analysis of PFS2/PFS3 may be interesting. #LCSM @OncoAlert 

#ESMO20 A big consideration is safety with lorlatinib. Note that 72% had grade 3/4 AEs and 5% with fatal AE. Dose interruption seen in about half of patients. AE leading to permanent discontinuation in only 7%. #LCSM @OncoAlert 

#ESMO20 The tornado plot shows some unique toxicities with lorlatinib including hyperlipidemia. Of utmost concern to me are the neurocognitive effects - noted in ~20% of patients. I feel these can be underreported, however, if recognized, they respond to dose reduction. #LCSM 

#ESMO20 Importantly, lorlatinib associated with better QoL scores - glad these are being captured. 
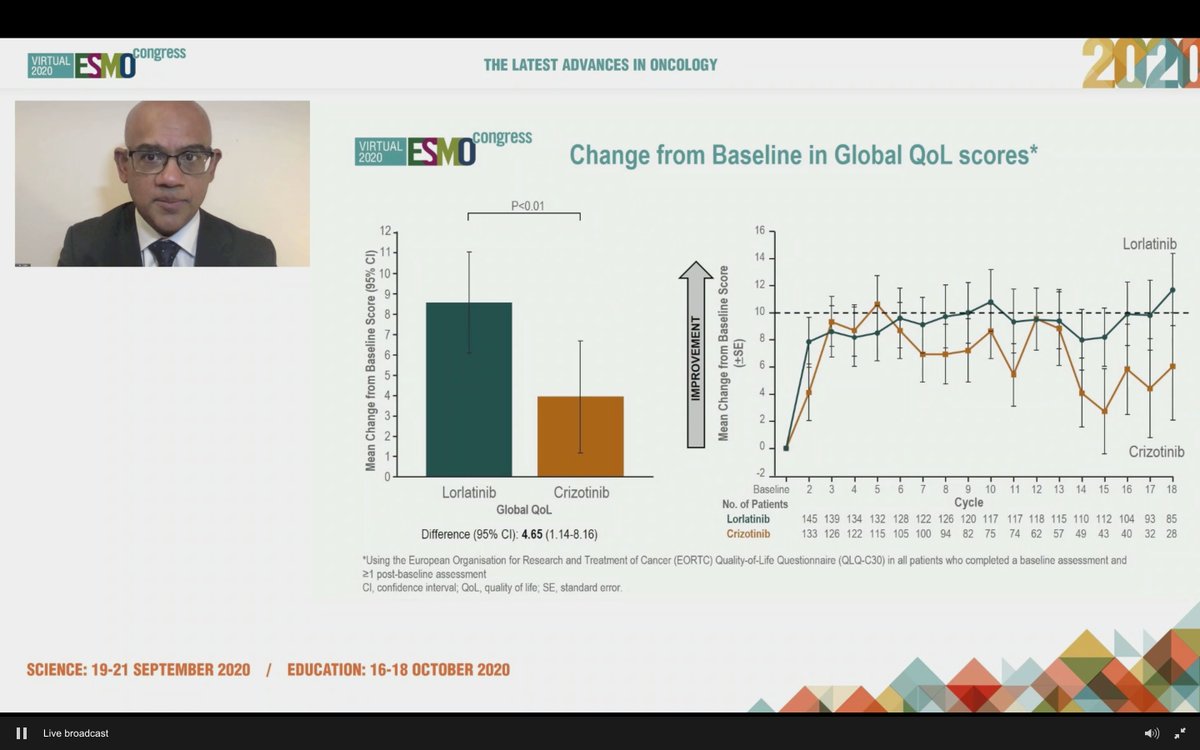
#ESMO20 We all expected lorlatinib to beat crizotinib but a HR of 0.28 certainly surpassed my expectations (like an ALK-DAURA). Seems like a new SOC but some hesitation given impact on sequencing and, most importantly, toxicity - esp neurocognitive impact. #LCSM @OncoAlert 

• • •
Missing some Tweet in this thread? You can try to
force a refresh