#ESMO20 Much anticipated results from CodeBreaK100: AMG 510 (sotorasib) in #KRAS G12C #NSCLC by @DavidHongMD building on exciting data seen over the past two years for this huge unmet need. #LCSM @OncoAlert @myESMO 

#ESMO20 KRAS G12C occurs in 13% of NSCLC, 3-5% CRC, and other tumors as well. Up until recently, considered "undruggable". Sotorasib is a highly selective KRAS G12C inhibitor that traps KRAS in its inactive GDP bound state. #LCSM @OncoAlert 

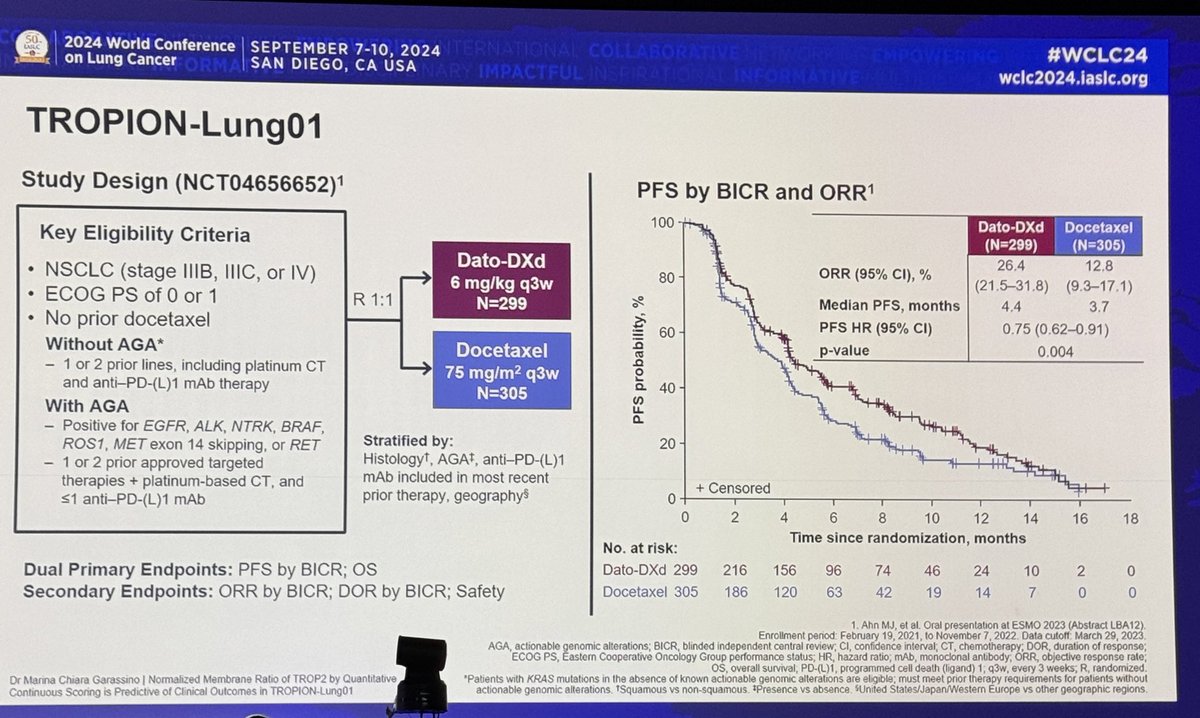
#ESMO20 CodeBreak100 is a phase I escalation/expansion trial of sotorasib monotherapy in KRAS G12C tumors. Primary endpoint was safety. Escalation established preferred dose of 960mg qday. #LCSM @OncoAlert 
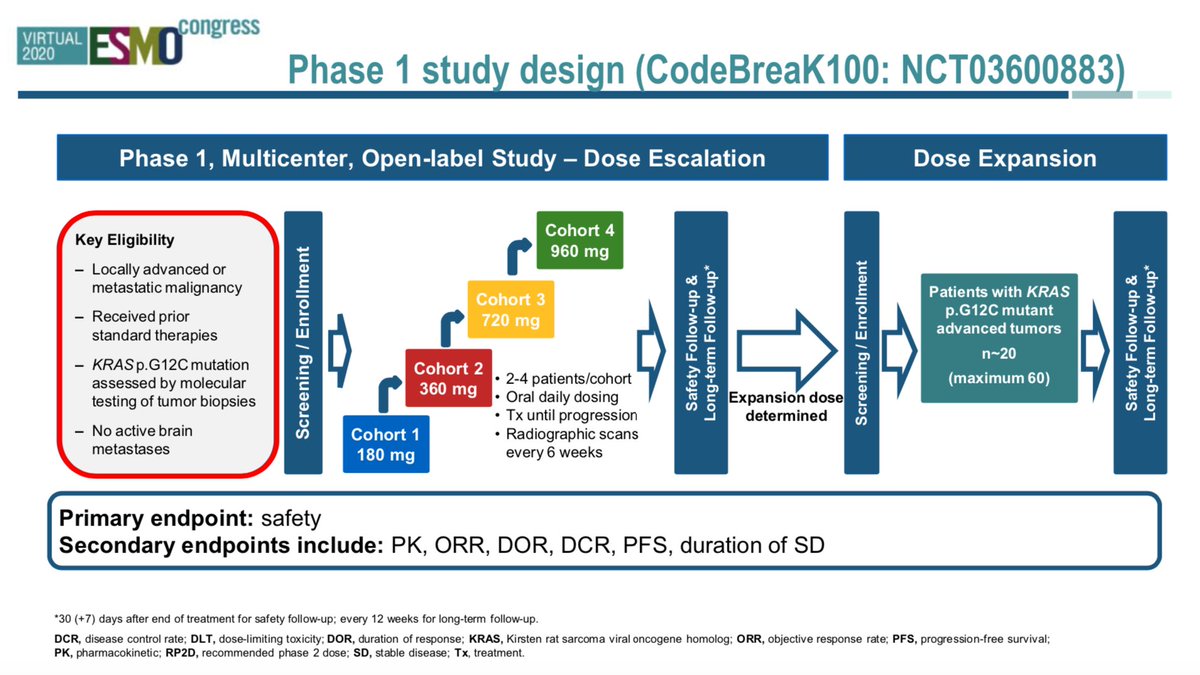
#ESMO20 Analysis here is on 59 patients with KRAS G12C NSCLC (34 were at the 960mg dose). Median f/u 11.7m. Mostly prior smokers, 90% with prior PD(L)1 therapy, fairly heavily pretreated (75% 3rd line or beyond) #LCSM @OncoAlert 

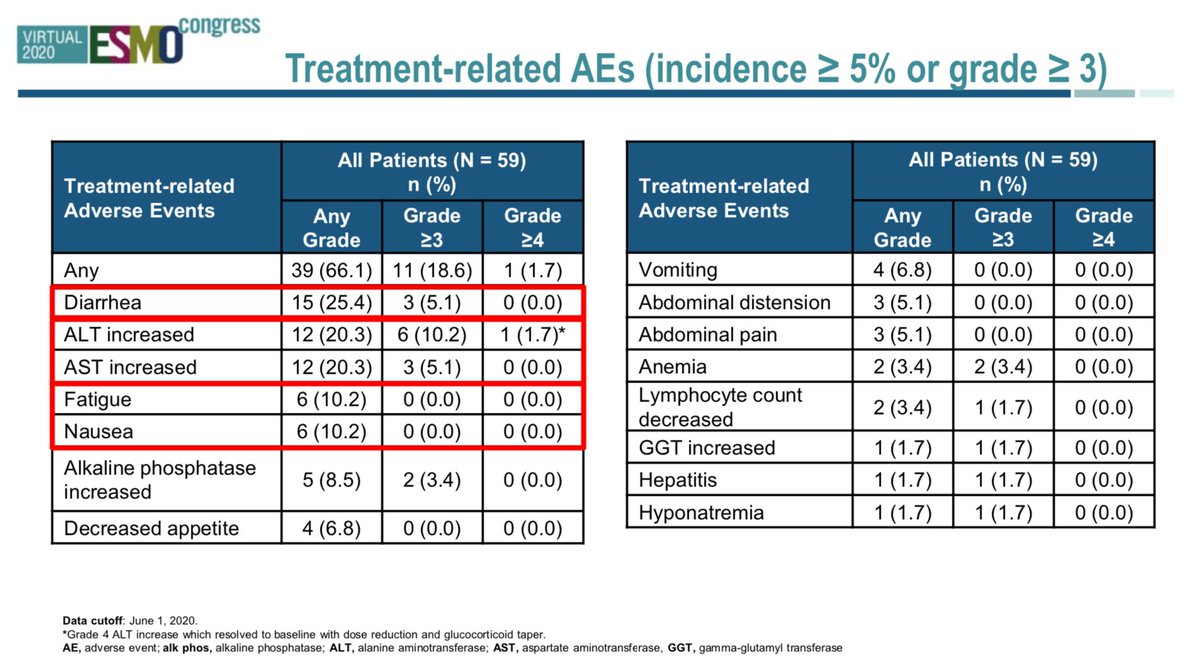
#ESMO20 KRAS G12C doesn't occur on normal tissue so the toxicity profile of sotorasib is very good. No DLTs. No fatal AEs. Only 1 patient stopped due to treatment related AE. #LCSM @OncoAlert 

#ESMO20 Few G3+ adverse events. One case of grade 4 ALT elevation that improved with dose reduction/steroids. This is even more impressive when we recall that 90% had prior immunotherapy. Not seeing the toxicity of IO-TKI sequence we see in EGFR/ALK. Different TME in KRAS? #LCSM 
#ESMO20 Efficacy of sotorasib in KRAS G12C NSCLC. ORR 32.2% with some reduction noted in 71.2% of patients at 6 weeks and an overall DCR of 88.1% (91.2% at 960mg). ORR lower than earlier reports, as expected, but still high in this setting. High SD rate but how durable? #LCSM 

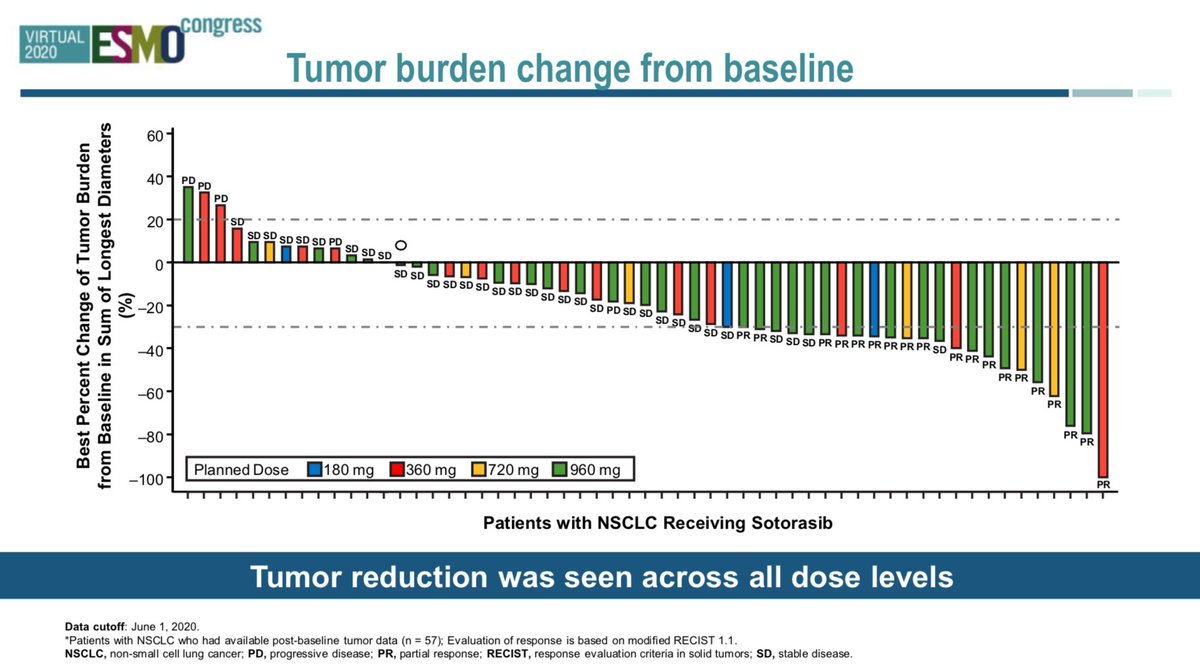
#ESMO20 Waterfall plot showing efficacy of sotorasib. Responses seen across dose levels, reassuring if dose reduction needed. ORR of 32.5% in a heavily pretreated population is impressive (docetaxel/ramucirumab ~ 23% for reference) with high SD rates (but how durable?). #LCSM 
#ESMO20 Median PFS with sotorasib in KRAS G12C NSCLC was 6.3m. Respectable in this setting but duration of response more impressive at 10.9m and long duration noted across doses. Median duration of SD was 4m which is not that long - casts the 90% DCR in a different light. #LCSM 

#ESMO20 Illustrative case provided in patient with STK11 co-mutation, 5 prior therapies since 2013 including chemo and nivolumab. Had CR in target lesions (low burden of disease shown) and had CNS response (though all CNS lesions had been radiated). Progressed at 1.5y, no DLTs. 

#ESMO20 Efficacy of sotorasib seen across KRAS G12C MAFs and TMB levels (by ctDNA). PDL1 predictive. Responses seen across co-mutations as well, though need larger numbers and need to correlate with duration/resistance, not just response. #LCSM @OncoAlert 



#ESMO20 Overall, sotorasib showing clear efficacy (RR 32.2%, DOR 10.9m, PFS 6.3m) and excellent safety profile. Would have a clear role today in salvage setting. Good safety profile makes it an appealing candidate for combinations in the 1L setting. #LCSM @OncoAlert 

#ESMO20 I think we had high expectations for the drug given early reports - but a RR of 35% with sotorasib is a clear win here. The reference is not alectinib/osimertinib, the reference is docetaxel. And given the high incidence of KRAS, the potential impact is huge.
• • •
Missing some Tweet in this thread? You can try to
force a refresh