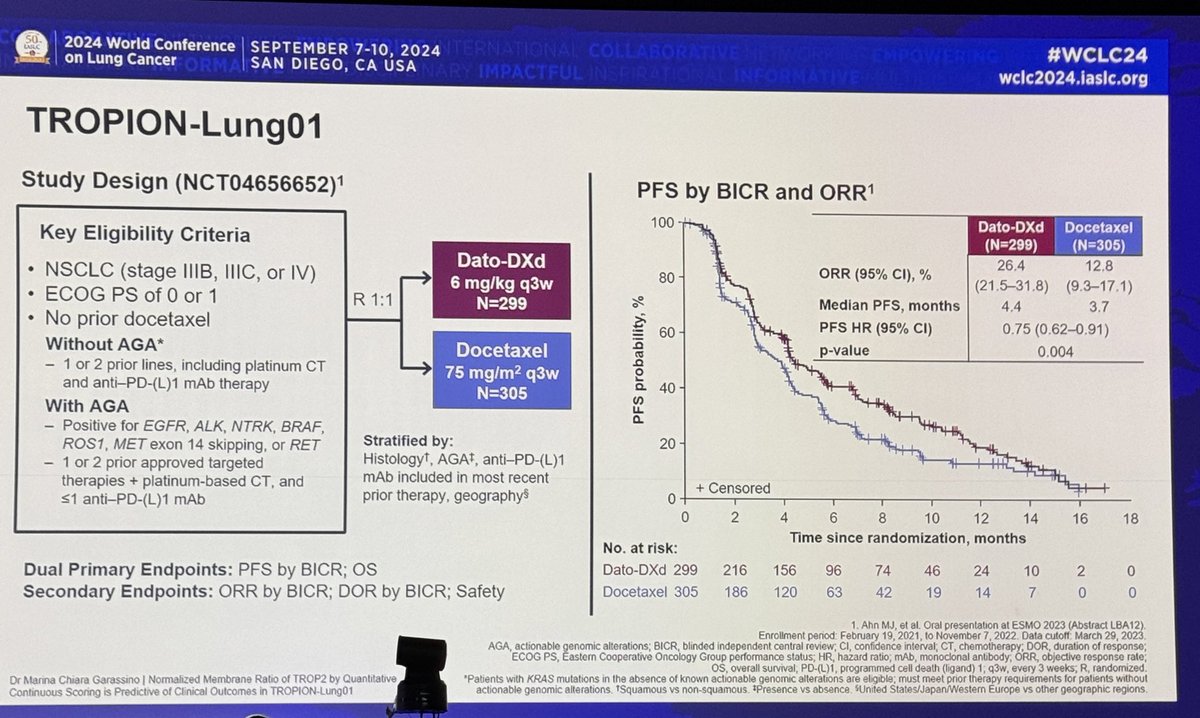
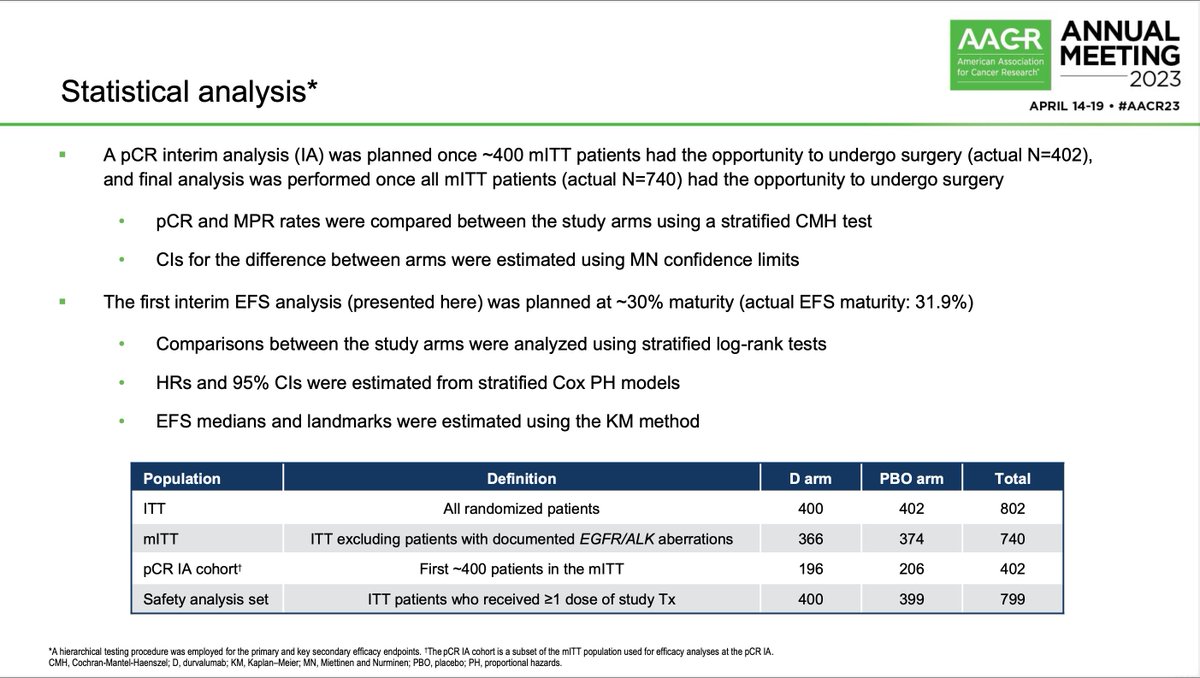
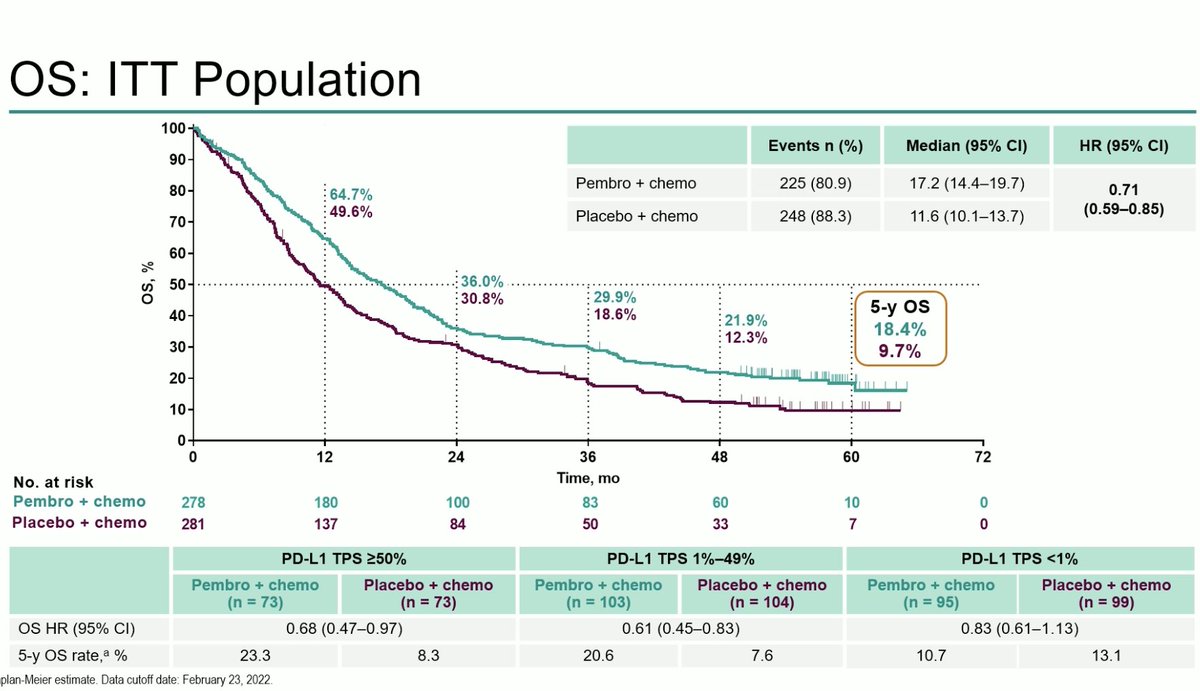
#ESMO20 Byoung Cho with important results from CHRYSALIS: amivantamab (JNJ-611863372, EGFR-MET bispecific) with lazertinib (3rd gen #EGFR TKI) in patients with sensitizing EGFR mt #NSCLC. Strong work from team including @Jbauml @AlexSpiraMDPhD @JSabari @RachelSanbornMD #LCSM 

#ESMO20 Both amivantamab (LOVE the phonetic spelling btw) and lazertinib show promising single agent activity in #EGFR NSCLC and amivantamab was granted FDA breakthrough designation for EGFRex20 NSCLC. Dual target inhibition with antibody and kinase inhibitor explored here. #LCSM 
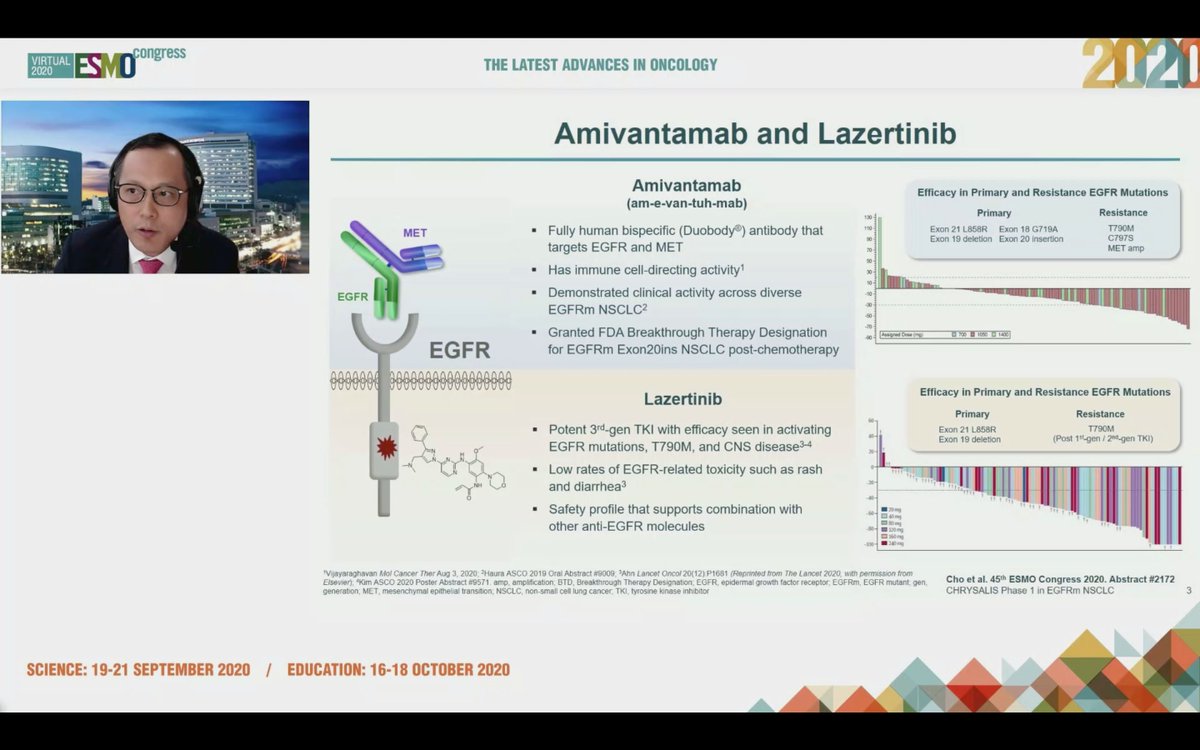
#ESMO20 CHRYSALIS is a phase I study with a dose escalation portion (established doses of amivantamab 1050mg or 1400mg based on weight given IV weekly then q2w with lazertinib 240mg once daily) and expansion in osi-resistant #EGFR NSCLC and treatment naive. #LCSM 

#ESMO20 Expected patient characteristics for this patient population - note 64% deletion 19 and 36% L858R mutations. Brain metastases noted in 37% of patients. #LCSM @OncoAlert 

#ESMO20 Safety shown here after median follow up of 6m. No DLTs noted in escalation. Very well tolerated with only 6% of patients stopping all therapy due to related AEs. One treatment related death - pneumonitis. G3 rash in only 4%. Infusion reactions common but mild. #LCSM 



#ESMO20 In osimertinib-resistant #EGFR+ NSCLC (major unmet need), amivantamab plus lazertinib had a RR of 36% and a clinical benefit rate of 60%, seen whether osimertinib given as first line or later lines and reductions seen after PD with lazertinib. #LCSM 
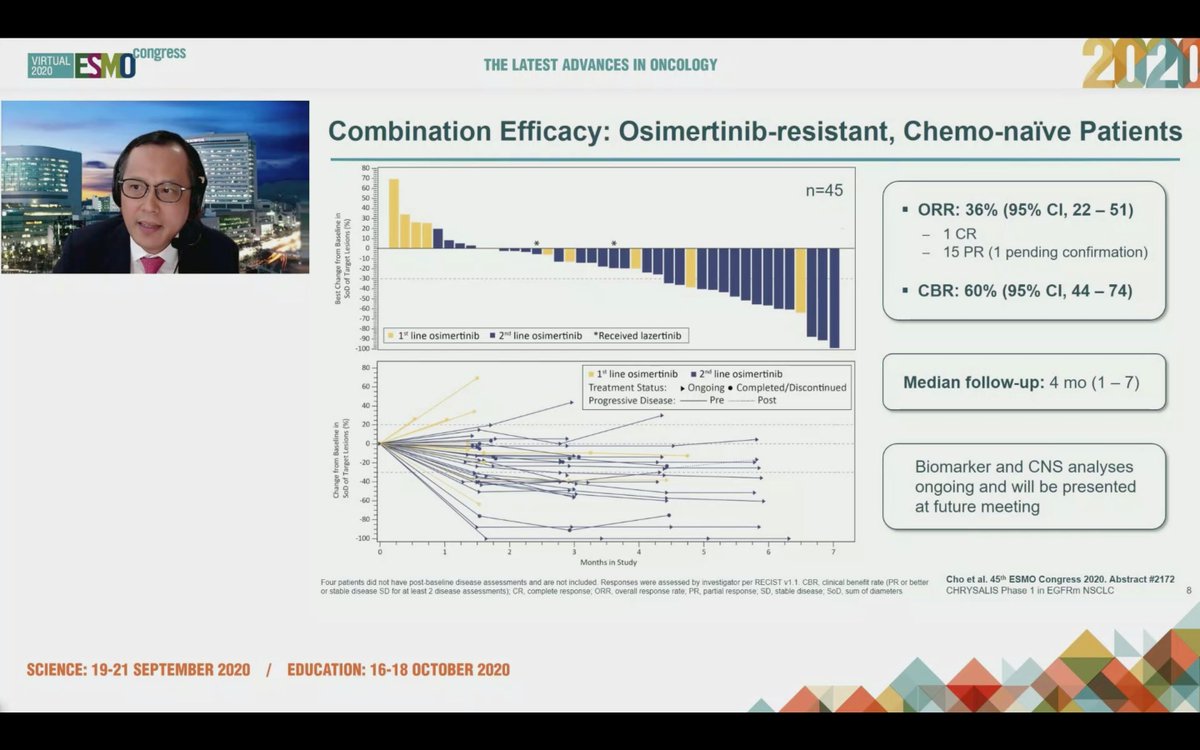
#ESMO20 Responses in this subset can be durable with 14/16 responses ongoing. Responses occurred early. This study is still immature and needs more follow up but very encouraging. #LCSM @OncoAlert 

#ESMO20 In treatment naive #EGFR NSCLC, amivantamab plus lazertinib had a RR of 100% (n=20). Spider plot below shows rapid and deep responses with a median time to response of 1.5 months. All responses still ongoing at 7m median f/u. #LCSM @OncoAlert 

#ESMO20 Very promising results with this combination. As a first line option, balance the need to receive IV infusions with a 100% RR but need more info on PFS and durability (and resistance). As a salvage option, this fills a major need today. #LCSM 

#ESMO20 While osimertinib is an excellent treatment option for #EGFR NSCLC, there is notable room for improvement on the 18m mPFS. The phase 3 MARIPOSA study will be an important one. Randomization to osimertinib, lazertinib, or lazertinib plus amivantamab. #LCSM 

• • •
Missing some Tweet in this thread? You can try to
force a refresh