1/THREAD - my presentation is kicking off at #EASD2020 about open source automated insulin delivery.
(You can see a full version of my presentation here: bit.ly/DanaMLewisEASD…, or read the summary below!)
(You can see a full version of my presentation here: bit.ly/DanaMLewisEASD…, or read the summary below!)
Note we should differentiate between open source (where the source of something is open), and DIY (do-it-yourself) implementations of open source code. Open source means it can be reviewed and used by individuals (thus, DIY or #DIYAPS) or by companies.
/2 #EASD2020
/2 #EASD2020

Open source automated insulin delivery (AID) has evolved since the first open source system, #OpenAPS, was made available in Feb 2015!
There are now three open source AID systems (OpenAPS, Loop, AndroidAPS) commonly used by the #DIYAPS community.
/3 #EASD2020
There are now three open source AID systems (OpenAPS, Loop, AndroidAPS) commonly used by the #DIYAPS community.
/3 #EASD2020

Many thousands of people have chosen to implement open source systems themselves for different reasons. We now estimate there are 29+ million hours of real-world experience with these open source systems.
/4 #EASD2020

/4 #EASD2020


But, it’s not just anecdotes - there is a lot of evidence around the use of open source systems, not only in the DIY community but also through a variety of studies and trials:
/5 #EASD2020

/5 #EASD2020


There are case studies, from adults and caregivers of children living with diabetes; physicians who use open source themselves as well as see patients using these systems; and also from athletic endurance events and also from those experiencing pregnancy.
/6 #EASD2020
/6 #EASD2020

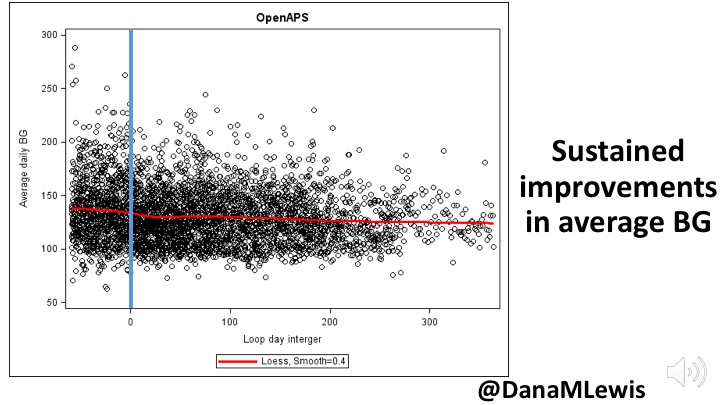
There are retrospective studies, some using #OpenAPS Data Commons (see: openaps.org/outcomes/data-…) and some gathering retrospective data directly in different countries. Whether overall or in a pediatric subpopulation, improvements in glycemic outcomes are observed.
/7 #EASD2020



/7 #EASD2020



There was also an in silico study of AndroidAPS (which uses a version of the #OpenAPS algorithm) done. (See paper here: liebertpub.com/doi/abs/10.108…)
/8 #EASD2020
/8 #EASD2020

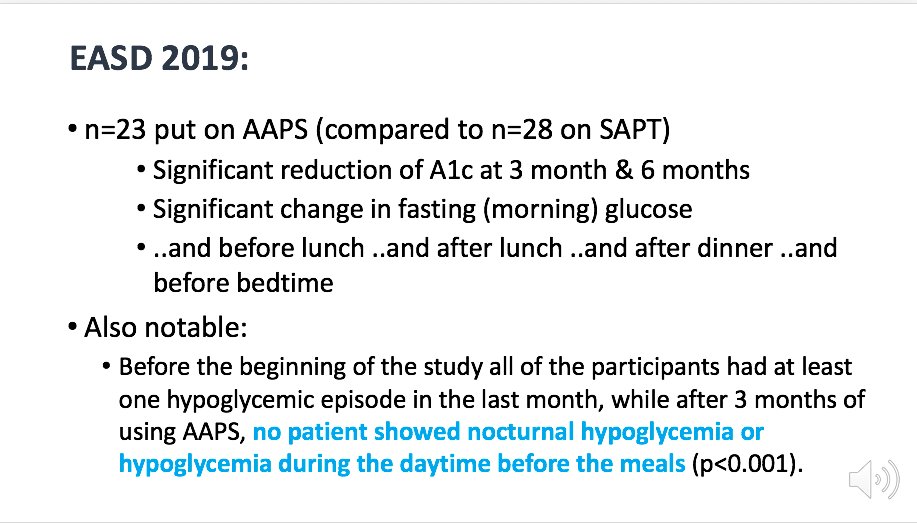
There was an observational study presented at EASD 2019 comparing an open source system (AndroidAPS) to sensor augmented pump therapy (SAPT). Other observational studies have also been done (results pending).
/9 #EASD2020


/9 #EASD2020


And, what everyone asks for: there’s a randomized control trial (CREATE) that is actively enrolling patients now in New Zealand. (Trial protocol here: rdcu.be/b4wUR)
So, there is a lot of existing and pending evidence around these open source systems.
/10 #EASD2020

So, there is a lot of existing and pending evidence around these open source systems.
/10 #EASD2020


It’s important to recognize when discussing open source and commercial AID systems that these are not always apples to apples comparisons: sometimes people are discussing DIY vs commercial, sometimes focusing on one component of an AID system rather than overall.
/11 #EASD2020
/11 #EASD2020
AID systems have the following components: an insulin pump, a continuous glucose monitor (CGM), an algorithm to drive changes in insulin dosing, and optionally interoperability to other devices (like smart phones or watches) that can interact or monitor the system.
/12 #EASD2020
/12 #EASD2020

Open source systems use already-existing pumps & CGMs, so most of the system that is open source is the algorithm and interoperable device (phone/watch) components. The open source algorithms have now been in use for almost 6 years, and have evolved significantly.
/13 #EASD2020
/13 #EASD2020

As I presented at #ADA2018 (bit.ly/DanaMLewis2018…), there are now numerous individuals doing no-bolus and/or no-meal entry/announcement, or a combination.
/14 #EASD2020
/14 #EASD2020
There are also features like autosensitivity (bit.ly/2018ADAautosen…) to respond to short-term changes in insulin sensitivity and autotune (bit.ly/2rMBFmn) to improve baseline settings.
/15 #EASD2020
/15 #EASD2020
Open source systems allow custom targets, including temporary targets, and use a concept of netIOB to improve the user’s understanding of what the system is doing (and why). It also generates and displays predictions of what the BG is likely to be in the future.
/16 #EASD2020
/16 #EASD2020
And, open source systems are interoperable with a variety of smart phones and watches, which can allow for easy input into the AID system as well as monitoring on the device of choice for the person with diabetes, their caregiver, and/or their loved ones.
/17 #EASD2020
/17 #EASD2020
This flexibility and choice is powerful. We do not choose to live with diabetes, but we deserve choices for the tools we use to help us live with diabetes. And, each of us may choose different tools - and these tools may change over time.
/18 #EASD2020

/18 #EASD2020


Not every person with diabetes will choose an open source solution (similarly, not all will choose to DIY). But some do, and others will innovate, develop, and contribute to these solutions, which often generate improvements to the diabetes community overall.
/19 #EASD2020
/19 #EASD2020

It’s important to remember that there is not one way in diabetes that works for everyone.
(Your diabetes may vary = YDMV), and your choices for which tools or how you use them for your diabetes will vary.
/20 #EASD2020
(Your diabetes may vary = YDMV), and your choices for which tools or how you use them for your diabetes will vary.
/20 #EASD2020

Some downsides of open source are conflated with DIY, and while those downsides (not regulatory approved; not covered by insurance; not in warranty) exist, the things we then describe as opportunities of commercial AID systems are not universally true.
/21 #EASD2020
/21 #EASD2020

(Commercial AID *should* be easy to use, easy to access...but regulatory approval does not mean that it is then affordable or accessible to all who want it. This, and the ability to choose separately pump, CGM, and algorithm for user, needs to be improved.)
/22 #EASD2020
/22 #EASD2020
A recent study (
/23 #EASD2020
https://twitter.com/danamlewis/status/1306295049542447110?s=20) highlights people want from commercial AID similar to what is desired from those implementing open source solutions: improved outcomes, flexibility in targets and the ability to correct for highs while protecting from lows.
/23 #EASD2020

Open source & commercial AID are not conflicting. We should ask: “What can we learn from open source to improve what’s available commercially?
Solutions from open source can help improve commercial systems; data from real-world usage can help solve new problems.
/24 #EASD2020


Solutions from open source can help improve commercial systems; data from real-world usage can help solve new problems.
/24 #EASD2020


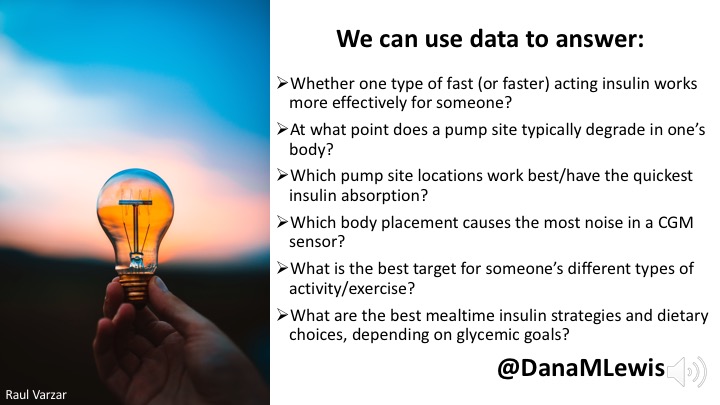
Open source AID has benefited from making small improvements quickly, and solving ‘small’ problems that add up to make a big difference.
We have the potential to take giant leaps to improve diabetes technology for all. Let’s do it, together.
25/25 #EASD2020
We have the potential to take giant leaps to improve diabetes technology for all. Let’s do it, together.
25/25 #EASD2020

• • •
Missing some Tweet in this thread? You can try to
force a refresh