1) SARS-CoV-2 Vaccines - I promised a Tweetorial and here we go. This is going to be long and nerdy. But I'll make sure it is easy to understand. If you want more details, please just read this: nature.com/articles/s4158…
2) I'll try to give an overview of the process, the technologies, correlates of protection, the candidates, how they perform in non-human primates and what we know about their performance in humans so far.
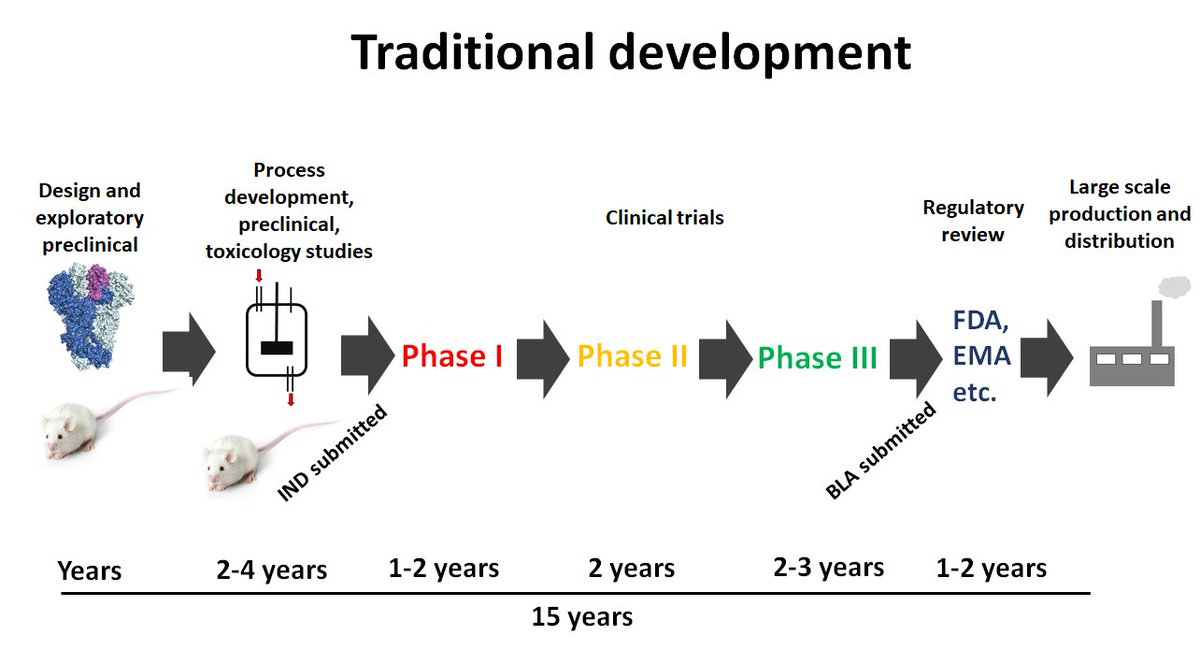
3) Let's start with the process. Developing vaccines usually takes a long time. Usually there is a medical need and some idea of how to design the vaccine, often in an academic lab. Versions of the vaccine are tested in iterative processes, the constructs are optimized....
4)....and this can take a few years. Then funding needs to be secured/a commercial partner needs to be found to advance this further into clinical trials. This can also take time. Once funding is in, a process is developed, GMP (good manufacturing practice aka high quality)....
5)...material needs to be produced, more formal animal experiments and toxicology studies are performed and then an IND (investigational new drug) application is filed. This process might take another 2-4 years. Then you go into Phase I trials (2 years), Phase II trials.....
6)...(2 years) and if everything looks great, the market is still there and the developer is sure the risk is low, they embarke on Phase III (which takes also about 2 years and is very very expensive). Just to explain the Phases: Phase 1 (<100 individuals) to check initial safety
7) and some immunogenicity, Phase II (a few 100 individuals) explores safety, immunogenicity and optimizes doses/regimens and Phase III (often thousands of individuals) looks at how well the vaccine works (efficacy) and safety in a large number of people.
8) Now, if Phase III looks good you file a biologics license application (BLA) to the FDA to bring the vaccine to the market. They may ask for more data, it is a process. You end up with about 15 years of development. Only then you start to produce the vaccine, which is expensive
10) Now, for SARS-CoV-2 this looks very different. A lot of preclinical work was done on coronavirus vaccines. The target, the spike protein was known. So, the fiddling around for years was skipped. This antigen was then just plugged into existing technology.....
11) ...and existing processes. In some cases, preclinical/toxicology data from similar vaccines was used for the initial IND. Clinical phases were staggered - which speeds thing up a lot.
12) Now, the question is, doesn't this compromise safety? Not really. Vaccine development is slow because it needs to be de-risked. You only go to the next step if you think the risk of failure - which will costs lots of money - is low. For SARS-CoV-2 money doesn't matter.
13) Everything is done at economic risk - and that speeds things up. No sane vaccine developer would do this in 'peace time'. So, we are already in Phase III trials, what happens next? Now, vaccines can be licensed the regular way or via an 'emergency use authorization'.
14) The 'emergency use authorization' allows the vaccine to be used before it is fully licensed based on available data that suggests a risk benefit. It is unclear if this will happen with SARS-CoV-2 vaccines, but it is possible. We will see.
15) The FDA published a guidance document for SARS-CoV-2 vaccine developers if you are interested. It can be found here. fda.gov/media/139638/d…
16) So, with considering all this, we end up with a vaccine development timeline of about 10 months to 1.5 years - depending on the licensing pathway. 

17) We will get back at what will happen once we have a licensed vaccine. But let me now go into the different types of vaccines that are around. We talk about 'vaccine platforms' when we talk about different kinds of vaccines. Currently, more than 180 vaccines are...
18) ....globally in development for SARS-CoV-2. Forty (40!!!!) are in clinical trials, ten (!!!!) are already in Phase III. The WHO keeps a living document with these which can be found here: who.int/publications/m…
19) I made a graph to visualize this (slightly outdated). It is just amazing. And these candidates, even the advanced ones are very much globally distributed. I'll explain in the end why this distribution and diversity is so important. 

20) But let's go through the platforms first. I feel people are a little afraid of different types of vaccines often because they don't understand how they work. So, let's change that.
21) We can divide the candidates into classic platforms (which are used for many viral vaccines), modern platforms (which are used for some newer licensed vaccines) and new platforms (which have never used for a licensed vaccine).
22) Let's start with classic: Inactivated vaccines are a typical example. You isolate the virus, grow it in cell culture (e.g. Vero cells) and then you harvest and concentrate it (usually by ultracentrifugation). Following that, you physically or chemically kill the virus....
23)....and you have your vaccine. This has been in use for a very long time and works for many vaccines (e.g. hepatitis A, influenza virus etc.). The virus can't infect your cells anymore but your immune system responds to it, mostly by making antibodies.
24) This can be done with SARS-CoV-2 but you need to have a biosafety level 3 production facility. Several vaccines in China, India and Kazakhstan made this way are being developed with some already far in Phase III. But since they are not developed in the US or Europe, it is ...
25) ...unlikely that they will be on the market here.
26) Another classic platform are live attenuated vaccines. Here, the virus is genetically weakened. In the old days a virus was just passaged under unfavorable conditions until it liked these conditions better than humans. Then you would inoculate humans and the virus...
27)....would just grow a little. Not making you sick but mimicking natural infection that triggers an immune response similar as to the pathogenic virus. Nowadays, there are more ways to do that, e.g. by altering the genetic code so that it doesn't translate well anymore...
28)....a technology called codon deoptimization, or by just taking away a gene of the virus that it needs to make us sick. However, coronaviruses are hard to manipulate genetically and there might still risk from these vaccines for people with compromised immune systems....
29) Historic examples for live attenuated vaccines are e.g. the measles or yellow fever vaccines, or FluMist, which is the flu vaccine that kids get as nasal spray. They work well. Unfortunately, only three live attenuated vaccines are in development for SARS-CoV-2....
30) ...and they are far behind.
31) OK, now modern vaccine platforms. Let's start with recombinant protein vaccines. For these you basically take the gene of a viral antigen and you express that antigen in a suitable system (e.g. bacteria, mammalian cells, insect cells, yeast or even plants).
32) No infectious virus is involved anywhere, making this very safe. For SARS-CoV-2 you can express the whole spike protein (like Novavax) or just the receptor binding domain (RBD) which it the part of the spike that docks to your cells or you can make virus-like particles.
33) Vaccines based on this technology work well and are on the market for influenza (FluBlok), hepatitis B and human papilloma virus (HPV). The technology works well and is safe. The frontrunner here is currently Novavax (just entered Phase III in the UK) and Sanofi.
34) Another modern technology are replication incompetent viral vectors. You basically take another virus, you gut its own genome and paste the gene for your desired antigen into it. Then you produce these vectors in a suitable cell line. Once they get injected into the vaccinee
35) they force some of the cells of the vaccinee to make the antigen. Again, in this case the antigen is the spike protein of SARS-CoV-2. Now, this is nothing that is concerning, in fact, SARS-CoV-2 does the same. The difference is, that the viral vectors do not replicate....
36)....but just deliver the genetic information for you cell to make the antigen which is then recognized by your immune cells. These types of vaccines are licensed for Ebola in the EU and have been considered safe for that purpose. For SARS-CoV-2 some of the vaccines in...
37)...Phase III trials are in this category including CanSino's vaccine which is based on an adenovirus 5 vector and AstraZeneca's vaccine which is based on a chimpanzee adenovirus vector. Adenoviruses are typically cause common colds and GI tract infections but these vectors....
38)...can't replicate and therefore are not pathogenic. The problem with the vectors is often, that humans have pre-existing neutralizing antibodies which might intercept the vector before it enters your cells. This is a big problem for AdV5 (CanSino) but AstraZeneca circumvented
39).this by using a virus that is not circulating in humans. However, if you give the same vector twice, you can still run into that issue, even if you use a vector that is not prevalent in humans. The huge advantage of these vectors is that they drive very good T-cell responses.
40) Now, there are other vectors that are replication competent. They have not been genetically gutted but the gene for the antigen of choice has just been added or has replaced a gene from the original virus. Viruses used for this are usually viruses that don't cause disease in
41)...humans or vaccine strains. One such vaccine, again for Ebola and based on the vesicular stomatitis virus (which infects usually cattle) is licensed in the EU and was found to be safe. While there are no leading candidates for those yet for SARS-CoV-2, promising candidates..
42)....based on a measles vaccine strain have entered clinical trials. Several more are in the preclinical stage. These vectors are usually pretty immunogenic because they trigger innate immune responses when they replicate. But they can be problematic in individuals...
43)...with compromised immune systems. One way around this are inactivated virus vectors. These are virus vectors that express the target antigen (in our case the spike) and also display it on the virion. These viruses can be grown, purified and inactivated just like inactivated
44)...vaccines, but are of course safer to culture because they are usually harmless. One of these approaches is based on rabies (Bharat Biotech in India), another one is based on Newcastle Disease virus (NDV). The NDV vector is interesting because it can be produced....
45)...using the influenza virus vaccine production process for which there is a lot of free capacity globally. Peter Palese is working on this with PATH (more below). biorxiv.org/content/10.110…
46) And then we have the new vaccine platforms. The first one is DNA vaccines. Basically, the gene for the target antigen, in our case again the spike, is inserted into a DNA plasmid under control of a mammalian promotor. This can now be grown up in E. coli in enormous amounts..
47)...is very cheap and relatively stable. This technology has been used for a long time but hasn't led to an effective human vaccine yet. The plasmids is then injected and often an electric shock is applied (electroporation) to get the DNA into the cell of the vaccinee.
48) Actually, bringing it into the cell is not enough, it needs to enter the nucleus. Once the DNA is there, mRNA is made - similar to what the virus itself would do - and protein is translated and expressed and then recognized by the immune system. Candidates based on...
49)...this technology are in clinical trials for SARS-CoV-2 but results haven't been released yet and progress seems to be slow.
50) And then there is the absolute new kid on the block: RNA vaccines. They come in two flavors. mRNA vaccines are basically just mRNA that is delivered to the cells. In contrst, self-replicating RNA consists of usually viral replicons that regenerates itself and the gene...
51) ...for the target antigen, also making mRNA for the target antigen in the process. Both technologies are very similar and new. RNA needs to be delivered, but not to the nucleus, just into the cytosol, making this easier. Usually, the RNA is complexed with lipid nanoparticles
52)..which are then injected intramuscularly or intradermal. Once the RNA is in the cell, it is translated into target antigen, in this case spike protein which is then made by the cell and recognized by the immune system. Two of the front-runner vaccines in the West....
53)...are based on mRNA encapsulated in LNPs. They are developed by Moderna and Pfizer. This is a very cool and new technology. But because it is so new, there might still be kinks in terms of large scale production. Amazingly, these vaccines are made completely in vitro...
54)...with no living cells involved. One caveat that is already apparent is, that they need to be stored frozen which is a challenge for distribution in the US and certainly also in low and middle income countries.
55) Now, before I go into correlates of protection and the different candidates, I'll take a break and get myself a glass of Cabernet Franc.
56) OK, let's continue. Now, we have our vaccines lined up. What are we looking for? The majority of them are designed to induce immune responses to the spike protein. Antibodies to the spike can neutralize the virus and there is data from nonhuman primates and some very....
57)...limited data from humans that neutralizing antibodies can protect you. The caveat is, that we don't know yet how much neutralizing antibodies you have (quantity and quality matter - my lab is working on this with many others). So, neutralizing antibodies is what everybody..
58)..is looking for. But let's not forget T-cells which are also induced by natural SARS-CoV-2 infection (more CD4 than CD8) and which could aid in protection. So that's what we are looking for in vaccines: Induction of neutralizing antibodies and T-cells to - mostly - the spike.
59) For nonhuman primate experiments there is an additional readout. We can challenge these animals with virus and see if they can get infected and if yes, how the vaccine diminishes virus replication compared to control animals. There are other useful animal models like some...
60) ..mouse models, ferrets, cats and hamsters but most of the comparable data for leading vaccine candidates is in rhesus or cynomolgus macaques. So, we will start there.
61) Inactivated vaccine candidates by both Sinovac and Sinopharm have been tested in nonhuman primates (NHPs). The vaccines were grown in Vero cells, purified and inactivated with the chemical betapropiolactone.
62) Then animals were vaccinated 2-3 times with different doses of the vaccine. They developed OK neutralizing antibody titers 1:50-1:200. When challenged intratracheally, their lung was protected from virus but their upper respiratory tract was not.
63) This would suggest protection from disease, but not from infection (we will get back to that in the end). Also, since these are the first vaccines that I am describing, the assays to measure neutralizing antibodies vary widely and so do the virus challenge doses and
64)...how virus titers are measured after challenge. So comparisons need to be taken with a grain of salt. Sometimes with a spoon of salt. Anyways, the inactivated vaccines protected the lung of these animals and reduced but not eliminated virus in their upper respiratory tract..
65)...compared to controls. They did make OK levels of neutralizing antibodies after vaccination as well. So, the vaccine worked. Maybe not my favorite, but not bad either. We will discuss their human results below.
66) Here are the respective papers if someone wants to dig deeper: science.sciencemag.org/content/369/64… and sciencedirect.com/science/articl…
67) The AstraZeneca vaccine, ChAdOx1 nCOV-19, was also tested in NHPs. It was given once or twice at 2.4x10^10 virus particles. Animals developed OK neutralizing titers that were around 1:5-1:40 after one shot and 1:10-1:160 after two shots. Animals were then challenge via 4 (!).
68)...routes with a relatively high challenge dose of 2.6x10^6 TCID50. If the vaccine was given twice, the animals lungs were completely protected, if the vaccine was given once, the lungs were partially protected. Not much protection was seen in the upper respiratory tract.
69) I forgot, they also looked at T-cells and got a pretty good response. Here is the paper. Awesome work by @DrNeeltje and team at NIH's Rocky Mountain Lab. nature.com/articles/s4158…
70) The next vaccine is also a viral vector, but this time and adenovirus 26 vector also expressing the spike protein and developed by Janssen. This vaccine was tested by Dan Barouch's group. I really liked that they made different spike versions and tested them in parallel.
71) Animals were vaccinated once with the respective AdV26 vectors at a dose of 1x 10^11 virus particles expressing different spike variants. The best one was called S.PP which has two stabilizing prolines and the polybasic cleavage site deleted. We are going to...
72)...focus on this one since this is the one selected for clinical development. After one shot neutralizing titers of 1:100 were reached. T-cells were measured as well but were low. The animals were then challenged with 10^5 TCID50 (lower than for AZ). Amazingly, the S.PP....
73)...animals were protected from virus replication in the lower and upper respiratory tract after just one shot. Pretty cool but I am not sure one shot will work in humans. They are now in Phase III as well. nature.com/articles/s4158…
74) This brings us to the Moderna mRNA candidate mRNA-1273. NHPs were given the vaccine twice at two different doses (10ug or 100ug). After the second dose, the animals developed substantial neutralizing antibody titers in the 1:501-1:3481 range. I mean, that's high....
75) The animals also developed good CD4 and Tfh T-cell responses. They were challenged with 7.5x10^5 TCID50 of virus (much more than Janssen, a little less than AZ) and complete protection of the lung was observed in the high dose group. The high dose group also showed....
76) ...little virus replication in the upper respiratory tract. These was some, but really little. As we all know, this vaccine is in Phase III as well now. nejm.org/doi/full/10.10…
77) Finally, there is the Novavax data. Novavax uses recombinant spike adjuvanted with Matrix-M. They tested 2.5 or 25 ug of protein given twice in the NHPs. Neutralizing titers reached obscenely high 1:17,920 - 1:23,040. Upon challenge with 10^4 TCID50 (much lower than others)
78)...they got complete protection of both the lower and upper respiratory tract. The paper can be found here: biorxiv.org/content/10.110…. There is also some more data in baboons.
79) To summarize the NHP data: Different vaccines have different abilities to induce neutralizing antibodies. All vaccines protect the lung/lower respiratory tract but many only provide partial protection of the upper respiratory tract (we will get back to that).
80) Also, different assays used, different challenge doses , different species (rhesus vs. cynos) and different readouts for detecting virus make direct comparisons different. But If I had to choose based on the data I'd take the AdV26 as prime and the Novavax vaccines as boost😜
81) Now a short red wine break before we go into humans.
82) OK, lets continue with humans, the very special primates. I'll again go by candidate/vaccine company.
83) CoronaVac (inactivated SARS-CoV-2+aluminium hydroxide) by Sinovac. They published data from a randomized, double bling placebo controlled trial. 3 or 6 ug of vaccine were given twice, adjuvanted with alum. They used two intervals, either two weeks or four weeks.....
84) They achieved non-impressive neutralization titers in the 1:30 to 1:60 range. But since e.g. the 50% protective titer for influenza is 1:40, this might be enough, who knows. The vaccine also seemed pretty safe with very few side effects.
85) One interesting thing they did was to compare the neutralizing antibody response in younger adults and the 50-59 age group. The 50-59 group had marked lower immune responses. We will get back to that in the end. This vaccine is currently in Phase III. medrxiv.org/content/10.110…
86) Sinopharm has published human Phase I and II data for a very similar vaccine. They evaluated 2.5, 5 and 10 ug in a three dose regimen in Phase I and then 5 ug in a two dose regimen in a two and three week interval in Phase II. The results are very similar to Sinovac.
87) Neutralization titers post-boost reached 1:121-1:316. This vaccine is now also in Phase III. jamanetwork.com/journals/jama/…
88) CanSino published two data sets with its AdV5 vector expressing the spike protein. The vaccine was only given once at 5x10^10 or 10^11 virus particles. It induce OK T-cell responses but neutralizing antibody responses were mediocre at best with titers of 1:18.3-1:19.5.....
89) Preexisting immunity to AdV5 impacted negatively on the induced response, in a way this as expected. Since older people have more immunity to AdV5 it seemed to do worse in older people. This vaccine also had considerable side effects including:
90) Fever, fatigue and headache, injection site pain etc. Grade 3 adverse reactions (mostly fever) were reported in 9% of individuals in the high-dose group. Not pleasant but maybe also not problematic. These are typically side effects triggered by vaccines that induce...
91) innate immune response and the body responds how it would response to an infection, with interferon which makes us feel sick. These site effects are unpleasant but usually transient and not too concerning (they might be problematic in kids, more about that later).
92) AstraZeneca's ChAdOx1 nCOV-19 (non-rep chimpanzee AdV expressing S) has data from a phase I/II, single-blind, randomized control trial. Vaccine was given at 5x10^10 virus particles once or twice (in a small subset). The vaccine induced good T-cell responses.
93) Neutralizing antibody responses were measured in three different assays and appeared to be OK robust after one shot (1:50-1:100 range) with some increase after the second shot (>1:100). Similar to CanSino, there were quite a few side effects. One group was even given...
94) paracetamol to see if this would alleviate the side effects but it didn't seem to do much. The study design included as licensed vaccine as placebo and so the differences in reactogenicity became nicely visible. Again, there are no real safety concerns but interferon driven
95) side effects might be unpleasant. This vaccine is far in Phase III trials and will likely also become available via Serum Institute of India. thelancet.com/journals/lance…
96) I forgot something important about the CanSino vaccine. It is now widely used within the Chinese military and is also in Phase III trials.
97) Now, let's move to Moderna's mRNA-1273 mRNA-based candidate. Data from a a phase I, open-label, dose-escalation trial is available. Doses used were 25 μg, 100 μg and 250 μg in a prime-boost regimen. The vaccine encodes the spike with two stabilizing prolines.
98) The vaccine was given twice in a 4 week interval. After the first vaccination, not much neutralizing antibody was induced, but titers post boost were pretty respectable in the 1:339.7 and 1:654.3 range (that's good!). T-cell responses were also detected, especially....
99) CD4 T-cells. The safety profile was OK with solicited systemic events were reported in 33%, 67% and 53% of individuals after the prime dose and in 54%, 100% and 100% of individuals after the booster for doses of 25 μg, 100 μg and 250 μg, respectively.
100) There wasn't much fever after the first dose but it was reported in 40% and 67% of individuals after the booster at doses of 100 μg and 250 μg - which seems high. Again, these are interferon driven self-resolving side effect that are unpleasant but not dangerous.
101) This vaccine candidate is currently being evaluated at the 100 μg dose in phase III clinical trials in adults, including those in older age groups. nejm.org/doi/full/10.10…
102) The second frontrunner with an RNA vaccine is Pfizer. They did not just evaluate one in the clinic, no, they tested at least two. BNT162b1 encodes a trimeric RBD, BNT162b2 encodes a full length spike with the two stabilizing prolines. Pfizer initially published a paper on...
103) BNT162b1 only (below), but than published a comparison of the two vaccine types in older and younger individuals and I will focus on that data here. nature.com/articles/s4158…
104) So, they used BNT162b1 and BNT162b2 at 10, 20, 30 ug doses (BNT162b1 as also tested at 100ug but the safety profile wasn't good so it was dropped) in a prime-boost regimen with a three week interval. They also stratified data by age groups 18-55 and 65-85.
105) Neutralizing antibody levels were dose dependent with, but levels seemed to be comparable across the two vaccine groups. Similar to the Moderna vaccine, not much of a neutralizing response was induced by the prime but levels rose to respectable 1:70-1:360 in the younger ...
106)...age group and lower 1:40-1:80 in the 65-85 age group, very similar to what has been observed by Sinovac. The safety profile was similar to Moderna's vaccine. Side effects included fever, fatigue and chills, especially after the second dose. Interestingly, side effects....
107) were lower in the older age group. BNT162b2 was finally selected for further development. This vaccine is far in Phase III trials. medrxiv.org/content/10.110…
108) The last one to discuss is Novavax. Novavax is using a recombinant spike based vaccine that is produced in insect cells and adjuvanted with Matrix M. The available data is from a randomized, observer-blind, placebo-controlled phase I trial. Participants were given...
109) ....two doses (in a 3 week interval) of nonadjuvanted vaccine at 25 ug, of adjuvanted vaccine at 5 or 25 ug or a single dose of adjuvanted vaccine at 25 ug. The adjuvant use is a saponin-containing and called Matrix M. Nonadjuvanted vaccine let to low titers after the
110) second dose (1:41), but adjuvanted vaccine given twice at either dose let to very high titers in the 1:3000 to1:4000 range. Adjuvanted vaccine given once resulted in lower titers (1:128). Good CD4 T-cell responses were also detected. Tolerability was good and fever was rare.
111) This candidate just entered Phase III trials in the UK. The paper can be found here. nejm.org/doi/full/10.10…
112) There is one more candidate that has data, a AdV5/AdV26 prime-boost combination from Gamaleya Institute in Russia. This vaccine was licensed after Phase II trials which is in my opinion unethical. There are also some questions about the data in their paper, so.....
113) I'll let judge everybody for themselves. thelancet.com/journals/lance… and here a little bit of criticism: cattiviscienziati.com/2020/09/07/not…
114) Now, as with the NHP data, it is really hard to directly compare neutralization titers and T-cell results since different assays were used. Any comparison needs to be taken with a grain of salt. However, a certain picture in terms of immunogenicity and side effects emerges:
115) In terms of immunogenicity, inactivated and AdV5-based vaccines seem to rank the lowest, followed by ChAdOx1-based vaccines and mRNA vaccines, and finally adjuvanted, protein-based vaccines, which show the best performance.
116) Reactogenicity seems to be lowest in inactivated and protein-based vaccines, followed by mRNA vaccines, with vectored vaccines having the highest rate of side effects.
117) Now, in general all of this looks good and I am amazed how well and fast this moves. But I still want to discuss a few topics (and I assume there will be a lot of debate about them).
118) All frontrunners and most of the >180 vaccines in the pipeline are given intramuscularly/as injected vaccines. This route is good to induce IgG which is prevalent in the lower respiratory tract and helps to protect the lung, which is great.
119) However, these injected vaccines are poor inducers of mucosal antibodies in the upper respiratory tract which is mostly protected by secretory IgA1. This might lead to immunity that protects the lung (mild/no disease) but still allows for infection and potentially for...
120)...onward transmission of the virus. Natural infection or live attenuated vaccines induce mucosal immunity and live attenuated vaccines might be much better in inducing sterilizing immunity in the upper respiratory tract. By not developing live attenuated vaccines we.... 
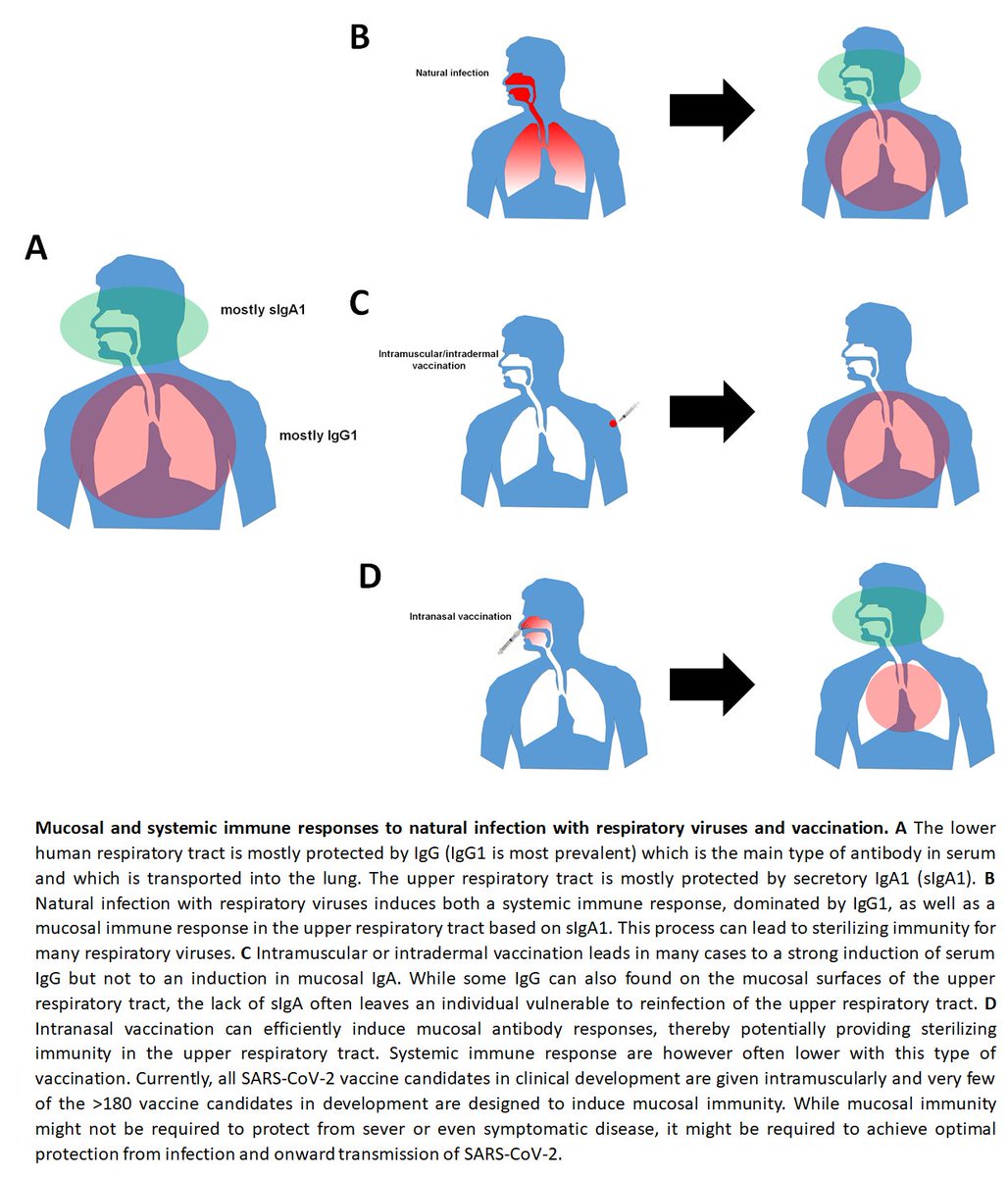
121) might end up with vaccines that protect us from disease but not infection and we might still be able to pass on the virus to others. This has been observed e.g. for influenza virus vaccines. Cool paper by @VivianaSimonLab about this below medrxiv.org/content/10.110…
122) The next issue is old people. Old people usually don't respond well to vaccines, as can be seen in the Sinovac and Pfizer trials. We also know this from flu. There are even special formulations of flu vaccines for old people that make them respond better. In addition,
123) it has even been shown that old people need much higher neutralizing titers than young people to be protected (again, for flu). So, the most vulnerable might profit the least from vaccines and we might needs special regimens or dosages for them.
124) The next issue are kids. Many vaccines that are far in the pipeline (AZ, Moderna, Pfizer) show high reactivity including fever in adults. Children usually respond worse than adults and some of these first vaccines might not be tolerable in children.
125) It is also entirely possible that the initial vaccines are effective and will be widely used but will then be replaced over time by vaccines that are as effective but less reactogenic.
126) We also have no clue how long vaccine induced immunity will last. For natural infection, we seem to see a relatively normal immune response. Vaccine induced immunity might be shorter or longer lived. If it is short lived, it would still be OK since booster doses can be
127) given. In fact, booster doses every few years are given for many vaccines, so I do not see this as an issue.
128) Another problem is doses needed. It is very unlikely, that one dose is enough. Given the current global population, this means we need 16 billion doses if two shots are needed. It is impossible for one vaccine producer to make that much vaccine. This is the reason why...
129) I am very happy that so many different vaccines and in so many geographic region are moving forward. This is the only way the demand can be met. Even then we might run into issues due to shortages of trivial things like glass vials, rubber stoppers or syringes.
130) Also, many of us have a 'Western' view on SARS-CoV-2 vaccines. It is very likely that vaccines from China, India and others will satisfy the global demand, not US or European companies. Currently, it is even hard to imagine how Moderna's or Pfizer's vaccines could be....
131) distributed in low and middle income countries given that they need to be stored frozen. Even within countries, it is often not clear yet, how vaccines will be distributed and who will get them first. The NAS has recently published a working document on this for the US....
132)...which can be found here: nationalacademies.org/our-work/a-fra…
133) And even if every vaccine now in advanced trials works and is churned out at full capacity, it will still take years to vaccinate the majority of the global population (I am not even taking vaccine hesitancy into account here).
134) Despite all the challenges discussed here, we are in the process of developing vaccines as a countermeasure against SARS-CoV-2 at an unprecedented speed, and it is certainly possible that vaccines with safety and efficacy that has been proven in phase III trials....
135).... might enter the market in 2020. Maybe initially not in large numbers and just for high risk groups but it will be a start. And every day after that will bring us closer to our normal way of living. I am very positive about this, we can do it!
136) PS: There was an important question about safety post licensure. This is normal now and is called Phase IV. Safety will be monitored even after the vaccine is on the market. This allows to find rare side effects.
137) PPS: Again, this is based on a review I recently wrote. Read that if you need more infos or refs. nature.com/articles/s4158…
138) OK, somebody is going to ask which vaccine I would take. After seeing all the human data I would likely go with Pfizer or Novavax. But that might change, based on data coming up from Phase III trial. I am also kinda curious about Sanofi.
• • •
Missing some Tweet in this thread? You can try to
force a refresh