
Viruses, viruses, viruses and vaccines.
V5=3xancestral+1x bivalent+1xXBB
Professor at the Department of Microbiology
Icahn School of Medicine at Mount Sinai
85 subscribers
How to get URL link on X (Twitter) App



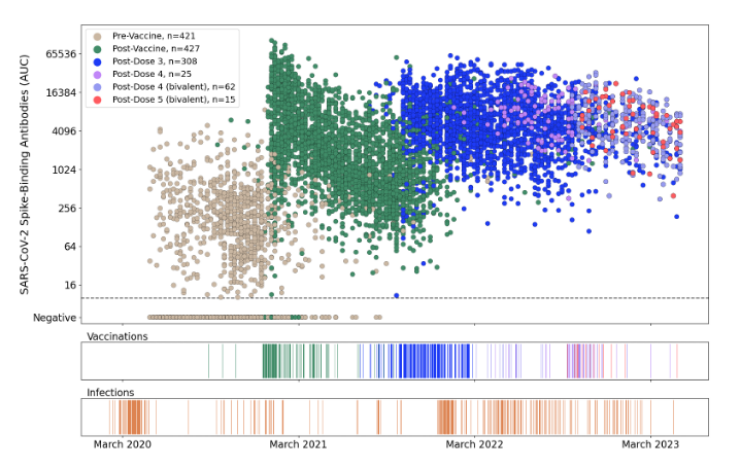




 2) We ran ELISAs with longitudinal samples from people who had received the primary vaccination series of COVID-19 mRNA vaccines including naive individuals (grey) and people who previously had SARS-CoV-2 infections (black).
2) We ran ELISAs with longitudinal samples from people who had received the primary vaccination series of COVID-19 mRNA vaccines including naive individuals (grey) and people who previously had SARS-CoV-2 infections (black). 

https://twitter.com/pucksandviruses/status/16364164632537825612) And climate change helps the mosquitos to move north, extending the potential range for viruses like dengue, chikungunya, Zika etc. This is also happening for some tick species, e.g. Hyalomma ticks in Europe which can carry Crimean-Congo Hemorrhagic Fever Virus.



 2) Here some links to the data: khub.net/documents/1359… and assets.publishing.service.gov.uk/government/upl….
2) Here some links to the data: khub.net/documents/1359… and assets.publishing.service.gov.uk/government/upl….