New: Two sets of documents we obtained show federal agencies approved and/or were aware of President Trump’s tweets prior to him publishing them.
The records provide a unique look into the workings of the president’s Twitter account.
The records provide a unique look into the workings of the president’s Twitter account.
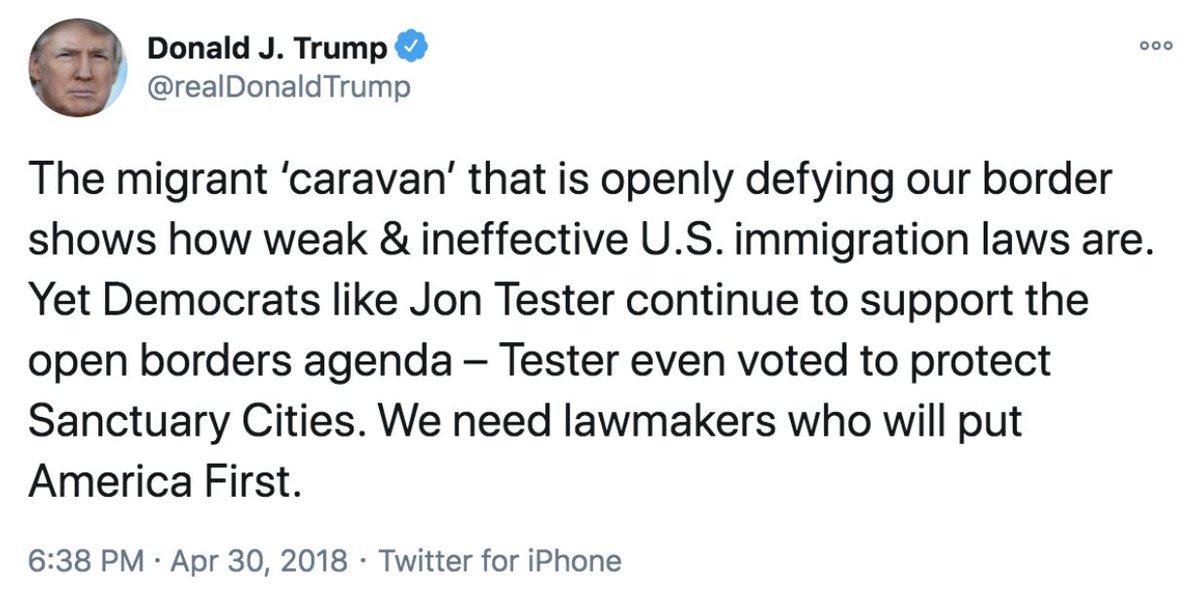
The first set shows DHS officials approved a tweet sent from President Trump’s personal Twitter account regarding the so-called migrant caravan on April 30, 2018.
americanoversight.org/document/cbp-r…
americanoversight.org/document/cbp-r…
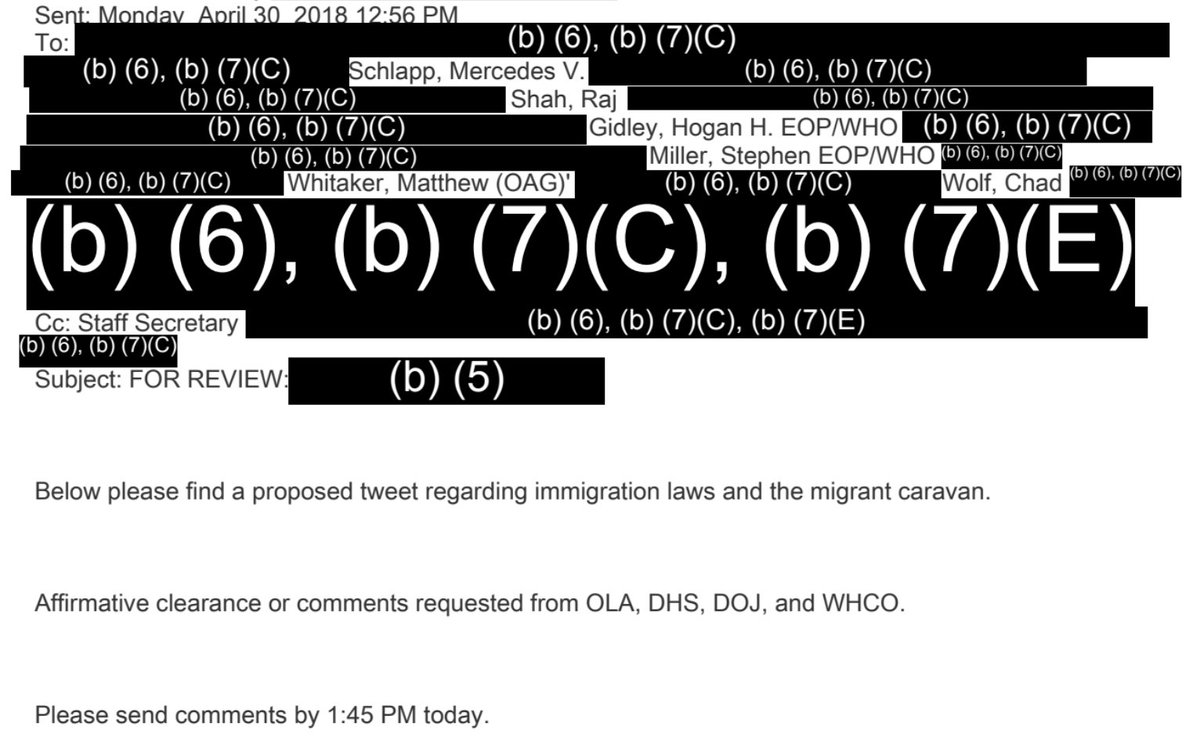
In an email titled “Urgent: [redacted],” an executive secretary at DHS requested “clearance ASAP, since White House deadline is 1:45 pm.”
That email was then forwarded to a group that included Stephen Miller, Chad Wolf, and others.

That email was then forwarded to a group that included Stephen Miller, Chad Wolf, and others.


“Below please find a proposed tweet regarding immigration laws and the migrant caravan,” wrote the redacted Staff Secretary before asking for comments before 1:45 PM.
At 1:12 on April 30, an unnamed official responded that the “Plcy clears.”
At 1:12 on April 30, an unnamed official responded that the “Plcy clears.”

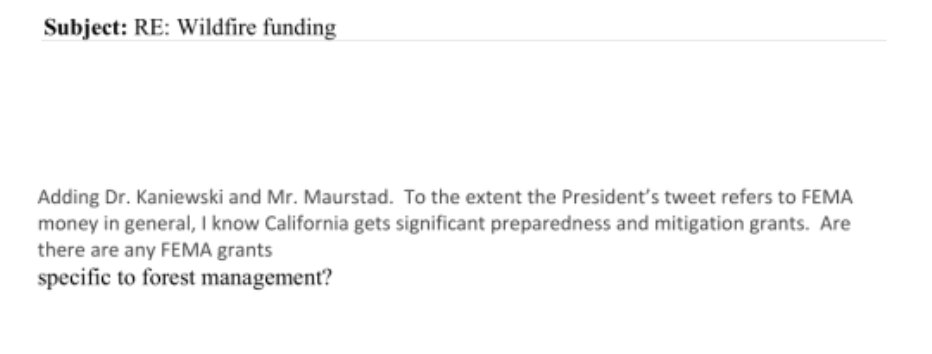
The second instance regards a tweet from January 2019. President Trump tweeted that FEMA should not send anymore money to California to help combat forest fires until “they get their act together.”
thehill.com/policy/energy-…
thehill.com/policy/energy-…
We requested communications about, linking, or referencing these tweets. The records we received in response suggest that FEMA was aware this tweet, or a tweet with similar content, would be published by the president.
americanoversight.org/document/fema-…
americanoversight.org/document/fema-…
After Trump tweeted about FEMA on January 9, 2019, Jessica Nalepa, then FEMA’s Director of External Affairs, sent an email to top FEMA officials: “Tweet is out.” She linked to an earlier version of Trump’s tweet that misspelled “forest.” 

In response to an inquiry from the Senate Appropriations Committee about the tweet, FEMA CFO Mary Comans asked top FEMA officials if it had been directed to stop recovery action. Eventually, the White House took over communications with press regarding the tweet. 


When the president’s tweets sow confusion or controversy, the posts are often depicted as the president’s “rogue” statements. DOJ has argued that both that his tweets are presidential actions and that they are not.
reuters.com/article/legal-…
reuters.com/article/legal-…
These documents show that sometimes the president’s tweets are vetted through a government agency and that agencies sometimes know about the statements in advance.
• • •
Missing some Tweet in this thread? You can try to
force a refresh