
Here it is: #LateBreakingAbstract at #ASTRO20 SS19 available to view now! Early analysis of trial (NCT03340974) using high-dose SBRT with avasopasem (GC4419) in #PancreaticCancer #PancSM #ASTRO20 Thread👇
This trial tried to address an unmet need for a specific group of #PancreaticCancer #PancSM patients whose disease had not progressed but remained unresectable after chemotherapy. #ASTRO20 @ACKoongMDPhD
Patients would be enrolled on the trial after finishing a minimum of 3 months of standard-of-care chemotherapy. They would receive SBRT with Avasopasem or a placebo control just prior to each SBRT treatment. #ASTRO20 

Avasopasem? TL;DR it’s a drug that converts the toxic free radicals created by RT and converts it to a form that normal cells can detoxify but tumor often cannot. In theory, less toxicity and more tumor killing with hypofractionation. #ASTRO20 @BrockSishc @MichaelStoryPhD 

The trial used an adaptive Bayesian design to assign the SBRT dose. The technical details are too much for @Twitter, but needless to say most patients got either 50Gy/ 5 fractions (EQD2=83.3Gy) or 55Gy/5 fractions (EQD2=96.3Gy). #ASTRO20 @colbertle
Between 2017-2019, we accrued 19 locally advanced patients @MDAndersonNews. When the trial expanded to @MoffittRadOnc @DukeRadOnc @DanaFarber_Hale and @UTSW_RadOnc in 2020, we completed the trial in 8 months! Testament to collaboration! #ASTRO20 @JessicaFrakesMD 

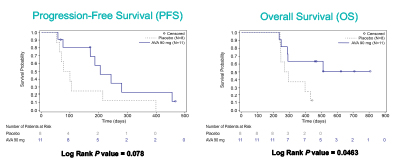
Early analysis in #PancreaticCancer patients with more than 1 year follow-up showed that SBRT + Avasopasem performed better than SBRT + placebo, but again too early to tell until the multicenter data mature. #ASTRO20 
Great responses in #PancreaticCancer: SBRT consistently produced radiographic responses in the primary tumors! Check out the waterfall plots. It seems that at around 6 months is when you see the best responses. #ASTRO20 @aguilera_md 

Surgery! This higher dose of SBRT did not preclude surgery. We had several patients with locally advanced disease go to the OR and nearly all patients got R0 resections without significant perioperative morbidity. #ASTRO20
Toxicity? No G4 or G5. 2 acute G3 GI toxicity events in each arm that were related to surgery or disease progression. A few late G3 GI events also likely related to local disease progression (yes even with this high dose). #ASTRO20
Significance? Too early to tell, but in carefully selected patients, high-dose SBRT, especially when combined with GC4419, has a promising early signal of benefit. #ASTRO20
Caveat 1: This is a small study with analysis at an early timepoint. Most of the patients in the trial do not have 1-year follow-up so this early positive signal may dissipate with time. #ASTRO20
Caveat 2: BR patients were included after expansion to a multi-center trial. Resected patients were censored from PFS analyses but were included in OS. The imbalance of imbalance of BR #PancSM between the arms needs to be accounted for before making any conclusions. #ASTRO20
Caveat 3: We do not have a way to distinguish which patients would benefit from consolidation with SBRT. Working on #Liquidbiopsy methods with @Aiims1742. A molecular marker to stratify patients would be a game changer! #ASTRO20
• • •
Missing some Tweet in this thread? You can try to
force a refresh


