Stock name – Indoco Remedies (#Indoco)
Bought now @ INR 312
Chart pattern – Around 3 years long horizontal BO on weekly closing above 317.40 (confirmation pending)
Technical target – INR 630
Time frame – Within 2 years
Stop loss – Will update once stock crosses INR 362
Bought now @ INR 312
Chart pattern – Around 3 years long horizontal BO on weekly closing above 317.40 (confirmation pending)
Technical target – INR 630
Time frame – Within 2 years
Stop loss – Will update once stock crosses INR 362
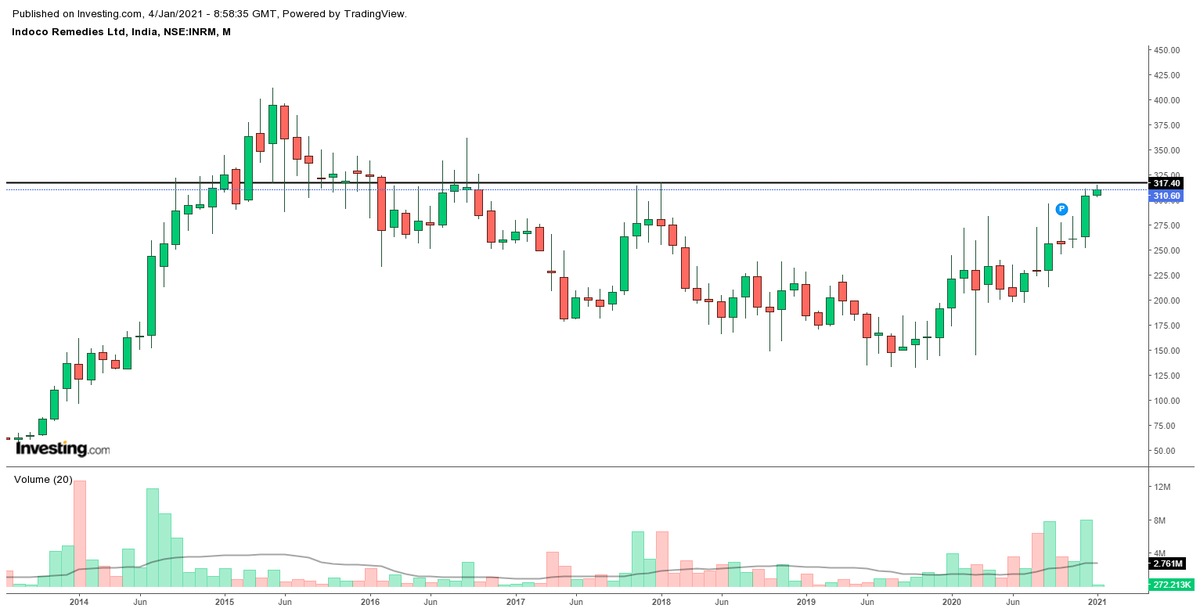
#Indoco - Press release dated 8th December 2020
Indoco wins major tenders in Germany for Allopurinol Tablets - bseindia.com/xml-data/corpf…
Indoco wins major tenders in Germany for Allopurinol Tablets - bseindia.com/xml-data/corpf…

#Indoco - Co. recently got USFDA approval for Apixaban Tablets.
Apixaban is an anticoagulant, or blood thinner. It is used for patients with health problems caused by a blood clot.
The US market size of Apixaban Tablets is USD 11,037 million as per IMS MAT June’20 data.
Apixaban is an anticoagulant, or blood thinner. It is used for patients with health problems caused by a blood clot.
The US market size of Apixaban Tablets is USD 11,037 million as per IMS MAT June’20 data.

#Indoco - Co. also launched FEVINDO (Favipiravir) 400 mg Tablets in India late Sept. 2020.
Fevindo ‐ 400 (Favipiravir) is an antiviral drug, effective against the RNA‐based influenza virus.
The drug has been approved by DCGI in the treatment of Covid‐19.
Fevindo ‐ 400 (Favipiravir) is an antiviral drug, effective against the RNA‐based influenza virus.
The drug has been approved by DCGI in the treatment of Covid‐19.

#Indoco - Headquartered in Mumbai, Co. is a fully integrated, research‐oriented pharma Company with presence in 55 countries. Indoco, a USD 145 million
Company, employs over 5500 people including more than 300 skilled scientists.
Company, employs over 5500 people including more than 300 skilled scientists.
#Indoco - The Company has 9 manufacturing facilities, 6 of which are for FDFs and 3 for APIs, supported by a state‐of‐the‐art R&D Centre and a CRO facility. The facilities have been approved by most of the Regulatory Authorities including USFDA and UK‐MHRA. 



#Indoco - Co. develops and manufactures a wide range of pharmaceutical products for the Indian and international markets. It generates more than 70 million prescriptions annually from over 3,00,000 doctors belonging to various specialties.
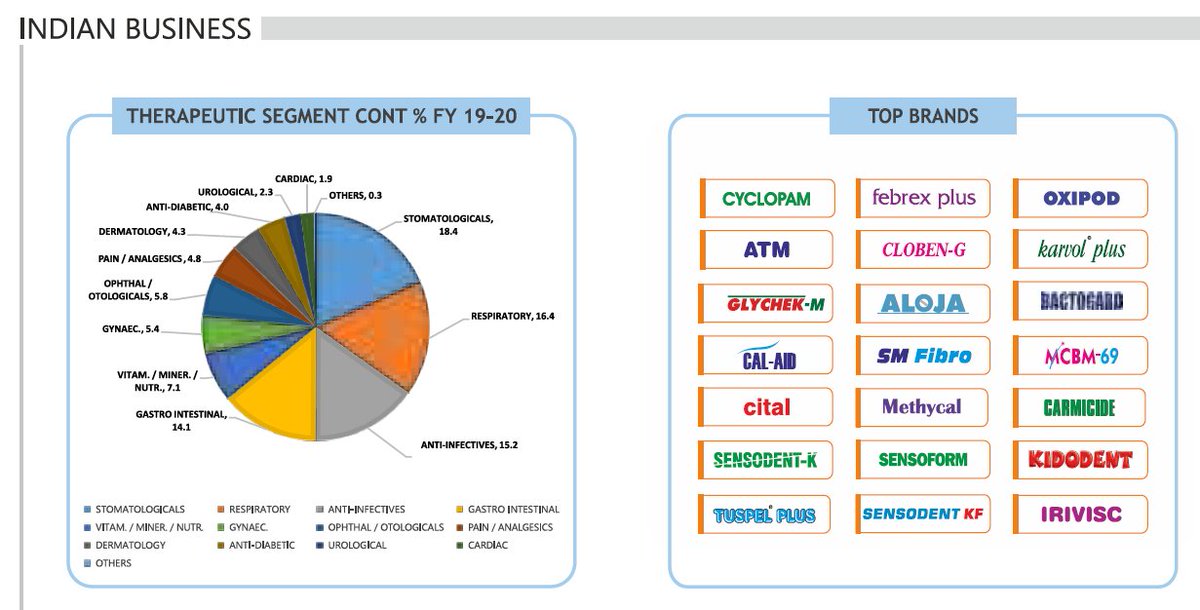
#Indoco - Co. has 9 domestic marketing divisions with a strong brand portfolio in various therapeutic segments including Respiratory, Anti‐Infective, Dental Care, Pain Management, Gastro‐intestinal, Ophthalmic, Cardiovascular, Anti‐Diabetics, Anti‐Obesity, etc.
#Indoco - Top Indoco brands include Cyclopam, Febrex Plus, Sensodent‐K, Oxipod, Cital, ATM, Cloben‐G, Sensoform, Sensodent‐KF, Karvol Plus, Glychek, Kidodent, Carmicide, Bactogard, etc. On the international front, Indoco has tie‐ups with large generic companies across the globe. 


#Indoco – Please read attached article for a detailed analysis of the stock.
Indoco Remedies – Multibagger in Pharma Formulations Growth Story?
finmedium.com/2020/10/indoco… via finmedium
Indoco Remedies – Multibagger in Pharma Formulations Growth Story?
finmedium.com/2020/10/indoco… via finmedium
#Indoco - UK to go into lockdown again till mid-February starting from Wednesday, due to new Covid strain releated cases.
Indoco is one of the largest suppliers of Paracetamol to UK.
Indoco is one of the largest suppliers of Paracetamol to UK.
#Indoco - Bought more today @ 312 to complete my desired quantity!
Refer attached buy note by ICICI Securities for more insights about the Company.
icicidirect.com/mailimages/IDi…
Refer attached buy note by ICICI Securities for more insights about the Company.
icicidirect.com/mailimages/IDi…

@AvadhMaheshwar2 - 👍
#Indoco - Future plan of action by the Company (excerpt from the Annual Report 2020) 

#Indoco - Technology absorption, adaptation and innovation by the Company (excerpt from the Annual Report 2020) 

#Indoco - With a good product mix of APIs in ophthalmics, anti-diabetic, anti-gout and other therapeutic categories, backed by DMFs and Certificates of Suitability (CEPs), the API division is well positioned to register an impressive growth in the coming years! 🤞 

#Indoco - Co. has strong R&D set-up & IPR! 



#Indoco - Stock has given a weekly closing above 317.40 as desired! Looking forward to a good monthly closing now...🤞 

• • •
Missing some Tweet in this thread? You can try to
force a refresh