
As promised, and after $CYDY corrected its erroneous eIND PR, I will discuss in this thread 3 topics:
1) Can its CD12 trial meet primary endpoint statistical significance?
2) Did the FDA obtain early access to trial data and why?
3) Questions for Cytodyn's wednesday conf. call
1) Can its CD12 trial meet primary endpoint statistical significance?
2) Did the FDA obtain early access to trial data and why?
3) Questions for Cytodyn's wednesday conf. call
1) Can its CD12 trial meet primary endpoint statistical significance?
We know from $CYDY SEC documents the following facts about its CD12 trial (for severe/critical COVID patients):
We know from $CYDY SEC documents the following facts about its CD12 trial (for severe/critical COVID patients):

We also reviewed 50+ research papers who ALL indicate that until May 2020, Standard Of Care for all severe/critical patients (excluding to be FAIR papers handpicking a specific subset like non intubated patients or ICU patients) until May had a mortality rate of 12-25% max.
$CYDY
$CYDY

Then we reviewed 20+ papers published afterward who ALL indicate that since Spring 2020 Standard Of Care mortality has been going down, due to a better understanding of medical signs and better care procedures.
One example from Nature (but there are many like that) 👇
$CYDY
One example from Nature (but there are many like that) 👇
$CYDY

So we wondered: can the $CYDY trial reach statistical significance for its primary endpoint (mortality at 28 days)?
There are only 2 unknowns: SOC arm mortality(20 to 25%), Leronlimab arm mortality (0 to 25%)
The rest is known: total patients, interim patients, interim deaths
There are only 2 unknowns: SOC arm mortality(20 to 25%), Leronlimab arm mortality (0 to 25%)
The rest is known: total patients, interim patients, interim deaths
To simplify it for non-stat guys, we loop through every possible combination of SOC arm and $CYDY Leronlimab arm mortalities, and for each combination compute:
- How is it likely to have produced the interim report 45/195 deaths
- Could it reach significance with all 390 patients
- How is it likely to have produced the interim report 45/195 deaths
- Could it reach significance with all 390 patients
This is very simple: for example the scenario the MOST positive for $CYDY is 0% Leronlimab mortality and 25% SOC mortality. But it is VERY unlikely. Because the combined mortality shall have been 8.3%, resulting in 16 interim deaths for interim instead of the 45 we got
On the other hand this extreme scenario would VERY PROBABLY reach statistical signiificance for the full trial 390 patients, as the difference between $CYDY Leronlimab and SOC is so large that it is VERY probable that the primary endpoint would be reached
Weighting all scenarios by their likelihood, we reached the probability that $CYDY primary endpoint reaches stat. significance at 5% is 1.2%
This is rough for statistical reasons (I didn’t take into account corrections for test multiplicity and started from a flat bayesian prior)
This is rough for statistical reasons (I didn’t take into account corrections for test multiplicity and started from a flat bayesian prior)
But this rough estimate (limited by $CYDY disclosed data) shows betting that the CD12 trial will succeed is a VERY HARD bet. The odds are stacked against Leronlimab. Where did it come from?
Simple: the interim deaths (45/195). Even if you believe Leronlimab works, this…
Simple: the interim deaths (45/195). Even if you believe Leronlimab works, this…
…deaths makes it incredibly unlikely that $CYDY Leronlimab with 2/3rd of patients had a low mortality. Second reason is: even if Leronlimab had a small positive effect, there were not enough patients post-interim (only 195) to demonstrate it. Diluted by pre-interim (bad) stats
This may explain why $CYDY DSMC asked to have a new interim at 75% to perform a (I quote the CEO, Oct.20th) «sample size reassessment». This is a technical term statisticians know well: …
it means the $CYDY DSMC wanted to use the 75% interim to reestimate the # of patients needed to reach significance. Why would they want to do that when the trial is about to end? I see only one rational explanation: because they could not detect the supposed Leronlimab effect yet
This would also explain why the $CYDY DSMC also asked to look at a NEW endpoint, mortality at 42 days. That’s what you do when you don’t have enough patients data at 28 days. You beef up the number by extending the observation time frame.
Doing so could expose you to a LOT of challenges with the FDA, so you don’t ask that unless you have an issue to solve.
Anyway the conclusion of this first part is: $CYDY CD12 trial is facing an uphill (up-Everest?) challenge due to its really bad interim mortality data
Anyway the conclusion of this first part is: $CYDY CD12 trial is facing an uphill (up-Everest?) challenge due to its really bad interim mortality data
Second question for today is:
2) Did the FDA obtain early access to trial data and why?
$CYDY CEO says the FDA firewall statistician got it, between Oct. 20th and Nov. 7th

2) Did the FDA obtain early access to trial data and why?
$CYDY CEO says the FDA firewall statistician got it, between Oct. 20th and Nov. 7th


From published FDA procedures we know they are supposed, for safety reasons, to receive aggregate or detailed mortality data from the sponsor ( $CYDY here) whenever deemed necessary (for safety reasons):
fda.gov/media/94879/do…
fda.gov/media/94879/do…
If the sponsor had access to this FDA data between Oct 20th. and Nov 7th (that I dont know, that’s a question for $CYDY ), then it must evaluate if this is a SEC « material event » for its shareholders. For example bad mortality data may constitute a material even….
…event. Who is in charge within Cytodyn of SEC reports and as such should supervise this decision? That’s another question for $CYDY but based on their website it seems to be their CFO, Mike Mulholland: 

The exact same person who just dumped millions of shares during the Xmas/New Year break. But this sale was part of plan decided in advance, will say $CYDY supporters. I agree, so let’s look at the date where this plan was decided by the the CFO. It’s indicated in his SEC report: 
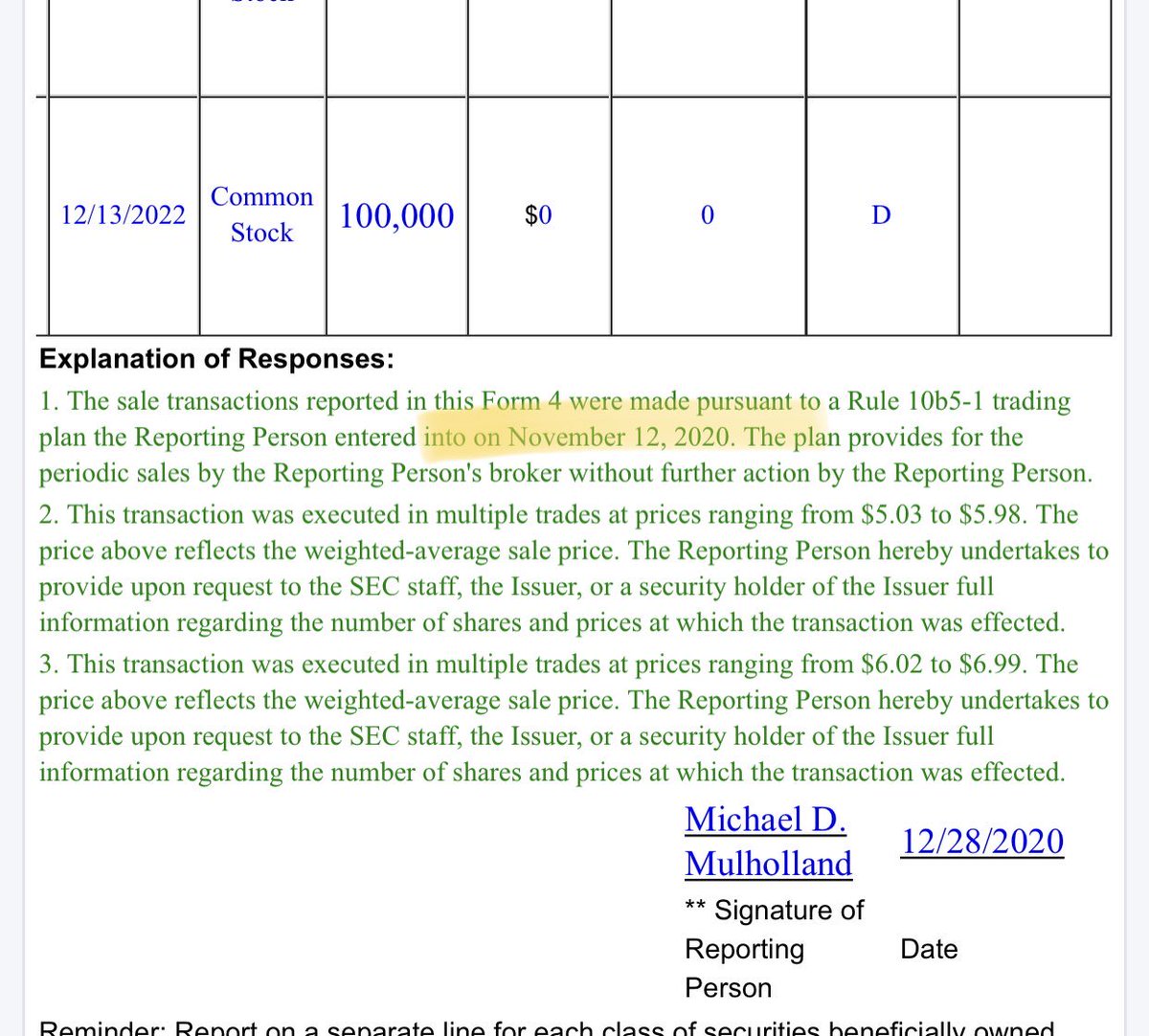
Said otherwise, Mike Mulholland, $CYDY CFO, decided to sell most of his stake in the company a couple days after unblinded CD12 data was transmitted to the FDA. However WE DONT KNOW YET if he heard of this data (even through a synthesis) or not. Only the company knows that
But we know safety data transmitted to the FDA as part of regular procedures can constitute a SEC material event and need to be evaluated as such. And we know he is within $CYDY the guy in charge of supervising SEC reports
That leads us to part 3:
3) Questions for Cytodyn's wednesday conf. call
We have first questions about the point above:
3) Questions for Cytodyn's wednesday conf. call
We have first questions about the point above:

Cc @cytodyn and @npourhassan1
If you have nothing to hide, the best you can do is answer these $CYDY questions during the conf call!
If you have nothing to hide, the best you can do is answer these $CYDY questions during the conf call!
• • •
Missing some Tweet in this thread? You can try to
force a refresh



