
TGA (Australian drug regulator) issued 2-year provisional approval for the Oxford-AstraZeneca vaccine, no age restrictions, with a cop-out on age - pushing the onus onto individual decisions. 4 to 12 week interval tga.gov.au/media-release/… ...1/n
...The Public Assessment Report released with the decision (49 pages) tga.gov.au/auspar/auspar-… It's for the AstraZeneca version: when CSL (local producer) is ready to supply, that won't need new clinical data... 2/n
...Reproductive safety study in mice is still underway - not recommended for pregnant women before then. Under clinical data, raised issue of follow-up injections possibly decreasing immunity... 3/n 
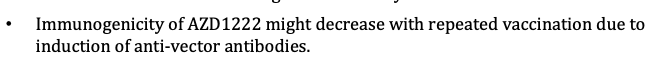
...Efficacy assessment is based on the now outdated November data, so it's just for UK & Brazil studies. The listed how many protocol versions the 2 UK plus Brazil & SA had...4/n 

...I hadn't picked up on this before (perhaps in EMA?). Rate of asymptomatic Covid-19 relied on self-swabbing & sending them in, TGA noted all participants didn't send them (no numbers). (Original paper said swabs from Scotland hadn't been analyzed yet: different issue.) ...5/n 

...TGA settled on: "Efficacy after a single dose is in the order of 60%", then goes into subgroup analyses by dosing interval. Then the report starts to get critical...6/n
...The clinical evaluator expressed concern about robustness of data & whether efficacy is high enough for use in Australia where little Covid-19. 💣 So: the vaccine is allowed to be used, but should it be?...6/n 

...On the subject of the second dose, the advisory committee stressed it was important: this report is transmitting multiple messages! Main message that's clear is concern about data robustness from all...7/n 

...Overall, not full-throated support for the vaccine. In acknowledging the problematic clinical trial basis, the TGA has opted for allowing the vaccine to be used, but recommending you really need informed decision-making at the individual level, because so much is unknown.../8 

• • •
Missing some Tweet in this thread? You can try to
force a refresh







