
Globally, we’re facing a severe COVID-19 vaccine shortage. Dose-sparing strategies could save lives. An argument against these strategies is that they might increase vaccine escape. We think these evolutionary impacts deserve a closer look, and could actually go the other way.
These ideas are from a collaboration with @mlipsitc, @danlarremore, @yhgrad, and are not yet peer reviewed. dash.harvard.edu/handle/1/37366…
First, we think most of the evolutionary action (selection) is happening at the population level, as different strains or variants compete for hosts. If vaccine escape is complete (efficacy = 0), escape strains replace other (WT) strains, and we’re no better off. 
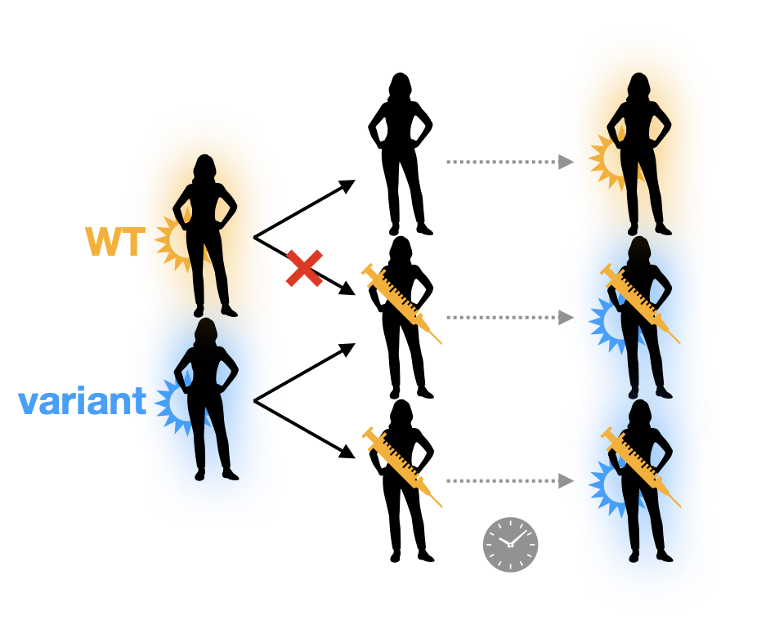
But if escape is not complete, e.g., some residual T or B cell immunity slows transmission of “escape” variants, then overall prevalence and incidence will drop. Aside from reducing disease, this could have some evolutionary benefits. 
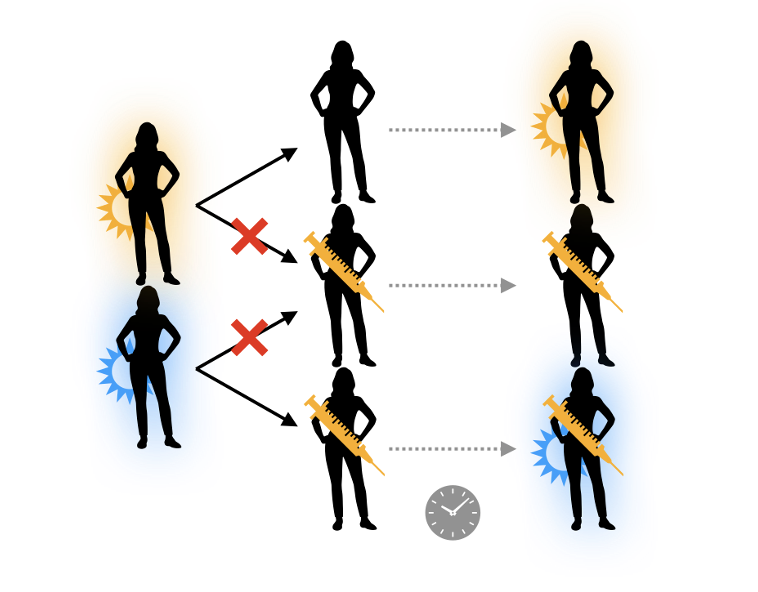
An obvious one is that with fewer infected hosts, fewer mutants will arise. Several lines of evolutionary theory further show that when populations are small and not quickly growing, selection on these mutants is less efficient.
In other words, the reduction in prevalence from vaccinating more people could slow the rate of vaccine escape and other types of adaptation. We found roughly this when modeling the impact of vaccination on the antigenic evolution of a flu-like pathogen. biorxiv.org/content/10.110…
But what about evolution within hosts? Adaptive immunity has a strong impact on viral evolution in chronic infections, e.g., of HIV and hepatitis C virus, and selects for escape variants.
We propose that immune selection is far less reliable in COVID-19 infections. From a founding population of a few virions, the population usually has only a few days to expand before transmission occurs. The antibody response can’t evolve much in this window.
Work by the @alauring lab showed, e.g., no selection in influenza viruses for vaccine escape in people who had received the seasonal flu vaccine. Diversity in typical COVID-19 infections seems similarly low.
In other words, we propose that even with one dose of a good vaccine instead of two, the expected within-host net viral adaptation rate is low (to the right of this curve) instead of at the top. There is some nuance here that seems tricky for Twitter. 
Furthermore, expanding vaccination coverage to more vulnerable people who might suffer longer infections could shorten their infections and reduce the rate of within-host escape from antibodies, and the generation of escape mutants.
It is impossible to develop a precise, predictive model of SARS-CoV-2 evolution at this stage. In the meantime, we have strong evidence that dose sparing could save many lives. We propose that it could have evolutionary benefits too.
And that should be @mlipsitch in the author list! Sorry, Marc.
And another typo... it's work by @LauringLab: journals.plos.org/plospathogens/…
• • •
Missing some Tweet in this thread? You can try to
force a refresh


