Pause of AZD1222 vaccine by some EU countries (Germany, Denmark, etc.) when UK has not raised any concerns, is relevant for India because > 85% of vaccine currently being admin in India is COVISHIELD (SII's AZD1222).
Thread on safety data from MHRA,UK
\1
Thread on safety data from MHRA,UK
\1
https://twitter.com/kakape/status/1372315648894844940
Up to 28 Feb 2021:
1st doses of COVID19 vaccines administered by UK
- 10.7 million of BNT162b2
- 9.7 million of AZD1222
& ~ 0.8 million 2nd doses mostly of BNT162b2.
AEFIs reported via Yellow card:
- 94809 of BNT162b2
- 201622 of AZD1222
- 843 unspecified
\2
1st doses of COVID19 vaccines administered by UK
- 10.7 million of BNT162b2
- 9.7 million of AZD1222
& ~ 0.8 million 2nd doses mostly of BNT162b2.
AEFIs reported via Yellow card:
- 94809 of BNT162b2
- 201622 of AZD1222
- 843 unspecified
\2

Up to Feb 28, 2021 in UK:
~10.1 million received Pfizer/BioNTech's mRNA vaccine, whereas 9.7 million received AZ/Oxford vaccine
Deaths reported via Yellow card:
- 251* of BNT162b2
- 275* of AZD1222
- 6 unspecified
% of deaths in vaccine recipients: AZD1222 > BNT162b2
\3


~10.1 million received Pfizer/BioNTech's mRNA vaccine, whereas 9.7 million received AZ/Oxford vaccine
Deaths reported via Yellow card:
- 251* of BNT162b2
- 275* of AZD1222
- 6 unspecified
% of deaths in vaccine recipients: AZD1222 > BNT162b2
\3
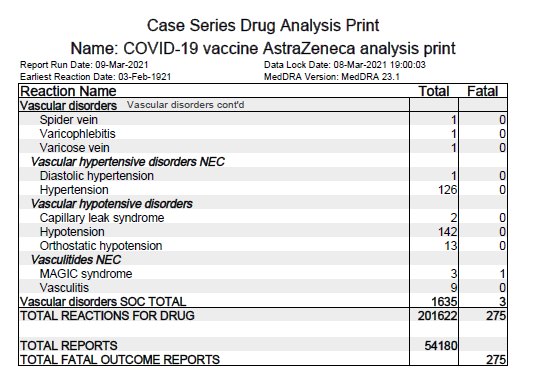


Blood disorders: 1098 @ AZD1222, 2294 @ BNT162b2
where, Thrombocytopenias: 35 @ AZD1222, 22 in BNT162b2
- Immune Thrombocytopenia: 22 (1 death) @ AZD1222, 9 @ BNT162b2
- Thrombocytopenia: 12 @ AZD1222, 13 (1 death) @ BNT162b2
- Thrombocytopenic purpura: 1 in AZD1222
\4

where, Thrombocytopenias: 35 @ AZD1222, 22 in BNT162b2
- Immune Thrombocytopenia: 22 (1 death) @ AZD1222, 9 @ BNT162b2
- Thrombocytopenia: 12 @ AZD1222, 13 (1 death) @ BNT162b2
- Thrombocytopenic purpura: 1 in AZD1222
\4


Cardiac disorders: 1922 (39 death) @ AZD1222, 1153 (26 death) @ BNT162b2
Deaths from
- Myocardial infarction: 12 @ AZD1222, 6 @ BNT162b2
- Myocardial ischaemia: 3 in AZD1222
- Heart failures NEC: 5 @ AZD1222, 2 @ BNT162b2
- Cardiac arrest: 13 @ AZD1222, 15 @ BNT162b2
\5



Deaths from
- Myocardial infarction: 12 @ AZD1222, 6 @ BNT162b2
- Myocardial ischaemia: 3 in AZD1222
- Heart failures NEC: 5 @ AZD1222, 2 @ BNT162b2
- Cardiac arrest: 13 @ AZD1222, 15 @ BNT162b2
\5




Eye disorders: 2150 @ AZD1222, 1398 @ BNT162b2
Gastrointestinal disorders: 22336 (5 deaths) @ AZD122, 10534 (12 deaths) @ BNT162b2
General disorders: 71732 (153 death) @ AZD122, 128915 (114 death) @ BNT162b2
Infections: 3839 (38 death) @ AZD122, 2059 (38 death) @ BNT162b2
\6

Gastrointestinal disorders: 22336 (5 deaths) @ AZD122, 10534 (12 deaths) @ BNT162b2
General disorders: 71732 (153 death) @ AZD122, 128915 (114 death) @ BNT162b2
Infections: 3839 (38 death) @ AZD122, 2059 (38 death) @ BNT162b2
\6


Immune system disorders: 542 @ AZD122, 528 @ BNT162b2
Metabolic disorders: 2644 (1 death) @ AZD122, 587 (1 death) @ BNT162b2
Muscle & tissue disorders: 24631 @ AZD122, 12823 @ BNT162b2
Nervous system disorders: 43951 (19 deaths) @ AZD122, 18059 (17 deaths) @ BNT162b2
\7

Metabolic disorders: 2644 (1 death) @ AZD122, 587 (1 death) @ BNT162b2
Muscle & tissue disorders: 24631 @ AZD122, 12823 @ BNT162b2
Nervous system disorders: 43951 (19 deaths) @ AZD122, 18059 (17 deaths) @ BNT162b2
\7


Psychiatric disorders: 3554 @ AZD122, 1409 @ BNT162b2
Reproductive & breast disorders: 297 @ AZD122, 389 (1 death) @ BNT162b2
Respiratory disorders: 5323 (11 death) @ AZD122, 3986 (14 death) @ BNT162b2
\8
------------------
Yellow card reports: gov.uk/government/pub…

Reproductive & breast disorders: 297 @ AZD122, 389 (1 death) @ BNT162b2
Respiratory disorders: 5323 (11 death) @ AZD122, 3986 (14 death) @ BNT162b2
\8
------------------
Yellow card reports: gov.uk/government/pub…


👉A comprehensive summary of key points from European Medical Agency's assessment of Thromobocytopenia.
Key confirmation from EMA's safety committee:
1. Benefits of AZD1222 (COVISHIELD) still outweigh the risks despite possible link to rare blood clots with low blood platelets.

Key confirmation from EMA's safety committee:
1. Benefits of AZD1222 (COVISHIELD) still outweigh the risks despite possible link to rare blood clots with low blood platelets.


2. No evidence of a problem related to specific batches of the vaccine or to particular mfg sites.
3. the vaccine may be associated w/ very rare cases of blood clots associated w/ thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it toclot) w/
3. the vaccine may be associated w/ very rare cases of blood clots associated w/ thrombocytopenia, i.e. low levels of blood platelets (elements in the blood that help it toclot) w/

or w/o bleeding, including rare cases of clots in vessels draining blood from the brain (CVST).
~20 million people in UK & EEA had got vaccine as of 16 March & EMA had reviewed only 7 cases of blood clots in multiple blood vessels (disseminated intravascular coagulation, DIC) &
~20 million people in UK & EEA had got vaccine as of 16 March & EMA had reviewed only 7 cases of blood clots in multiple blood vessels (disseminated intravascular coagulation, DIC) &

18 cases of CVST. A causal link w/ thevaccine is not proven, but is possible and deserves further analysis.
9 of DIC & CVST cases from member states resulted in deaths. Most of these occurred in people < 55 years & majority were women👩.
Difficult to look at background rate.
9 of DIC & CVST cases from member states resulted in deaths. Most of these occurred in people < 55 years & majority were women👩.
Difficult to look at background rate.

However, based on pre-COVID figures it was calculated
that < 1 reported case of DIC might have been expected by 16 March among people < 50 w/in 14 days of receiving vaccine, whereas 5 cases had been reported. Similarly, on avg 1.35 cases of CVST might have been expected among
that < 1 reported case of DIC might have been expected by 16 March among people < 50 w/in 14 days of receiving vaccine, whereas 5 cases had been reported. Similarly, on avg 1.35 cases of CVST might have been expected among

this age group whereas by the same cut-off date there had been 12.
Press brief on EMA assessment here: ema.europa.eu/en/news/covid-…
Information for vaccine recipients (left pic) and health professionals (right pic):

Press brief on EMA assessment here: ema.europa.eu/en/news/covid-…
https://twitter.com/das_seed/status/1372609822605123586
Information for vaccine recipients (left pic) and health professionals (right pic):


🚨 (Amended) Product Information of AstraZeneca/Oxford COVID19 vaccine (COVISHIELD if mfg by SII, India) as approved by CHMP, @EMA_News on 19 March 2021, pending endorsement by @EU_Commission: ema.europa.eu/en/documents/p…
Product Info of COVISHIELD in India also needs to be updated.

Product Info of COVISHIELD in India also needs to be updated.


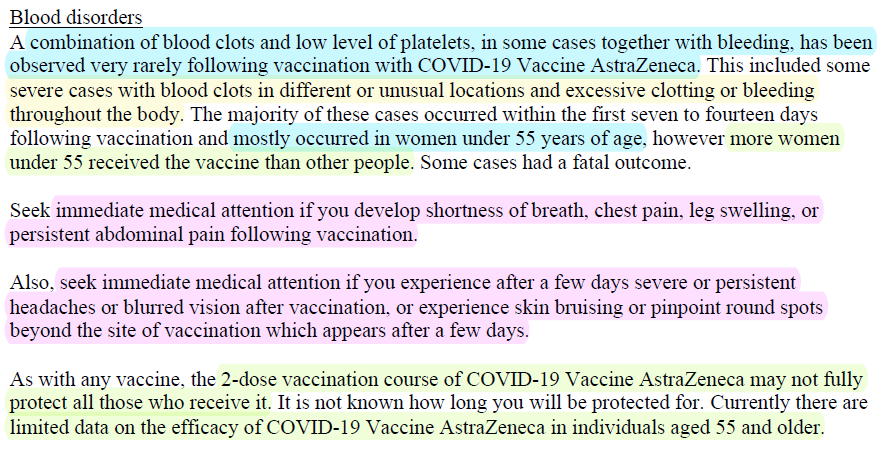
Seek immediate medical attention if AEFI like
- shortness of breath, chest pain, leg swelling, or
persistent abdominal pain
- severe or persistent headaches or blurred vision, or experience skin bruising or pinpoint round spots beyond site of jab which appears after a few days


- shortness of breath, chest pain, leg swelling, or
persistent abdominal pain
- severe or persistent headaches or blurred vision, or experience skin bruising or pinpoint round spots beyond site of jab which appears after a few days


Based on clinical/laboratory features of 9 patients in Germany & Austria, a study finds: AZD1222 is associated w/ development of prothrombotic disorder that clinically resembles heparin-induced thrombocytopenia but which shows different serological profile researchsquare.com/article/rs-362… 

At least 4 cases in India of thrombocytopenia post COVISHIELD, includes-
33yr/F (vaccine: 22 Jan, death: 3 Feb) w/ death cause earlier reported as "cerebral thrombosis" after post-mortem
80yr/M: AEFI- low platelets initially, later intracranial bleeding



33yr/F (vaccine: 22 Jan, death: 3 Feb) w/ death cause earlier reported as "cerebral thrombosis" after post-mortem
80yr/M: AEFI- low platelets initially, later intracranial bleeding
https://twitter.com/das_seed/status/1372220495412801538



Bijwerkingencentrum Lareb, Netherlands has received 5 reports of extensive thrombosis w/ a low platelet count following vaccination with Vaxzevria (COVISHIELD). It occurred 7 to 10 days after vaccination. These are women between 25 & 65 years old. lareb.nl/news/meldingen… 

Vaxzevria (COVISHIELD): As of 4 April 2021, 169 cases of CVST & 53 cases of splanchnic vein thrombosis reported to EudraVigilance.
🚨EMA has concluded that unusual blood clots w/ low blood platelets should be listed as very rare side effects of Vaxzevria.
ema.europa.eu/en/news/astraz…

🚨EMA has concluded that unusual blood clots w/ low blood platelets should be listed as very rare side effects of Vaxzevria.
ema.europa.eu/en/news/astraz…


A short commentary on @MHRAgovuk assessment of reported very rare AEFI: specific blood clots (thrombosis) with low platelets count (thrombocytopenia) associated w/ Vaxzevria (COVISHIELD).
1. Benefits of vaccine outweighs risk for vast majority of people. By 31 March, 20 million
1. Benefits of vaccine outweighs risk for vast majority of people. By 31 March, 20 million
doses have been give. MHRA had 79 case reports upto & including 31st March, 19 people died. All cases post 1st dose. Cases occurred in 51 women and 28 men, all in age group 18 to 79 years.
2. Risk of this rare blood clot about 4 people in a million. Among deceased, 3 out of 19
2. Risk of this rare blood clot about 4 people in a million. Among deceased, 3 out of 19
under 30 years. 4 out of 19 were of cerebral venous sinus thrombosis w/ low platelets, and 5 were other kinds of thrombosis in major veins.
3. Balance of benefits to risk is very favorable in older people & more finely balanced in younger people. Advise on how to minimize risk.
3. Balance of benefits to risk is very favorable in older people & more finely balanced in younger people. Advise on how to minimize risk.
4. Anyone w/ symptoms 4 or more days post vaccination should seek prompt medical advice, new onset of a severe or persistent headache or blurred vision, shortness of breathe, chest pain, leg swelling, persistent abdominal pain, or indeed unusual skin bruising or pinpoint spots.
• • •
Missing some Tweet in this thread? You can try to
force a refresh