
Quantum Information Theory.
Public health. AIDAN.
Live life with no regrets.
How to get URL link on X (Twitter) App


 ● Severe symptomatic COVID-19+ve: 93·4% (57·1–99·8) w/ 16 cases (1 COVAXIN, 15 placebo)
● Severe symptomatic COVID-19+ve: 93·4% (57·1–99·8) w/ 16 cases (1 COVAXIN, 15 placebo)


https://twitter.com/das_seed/status/1409836073509863426
 FAQ by @MoHFW_INDIA circulated among designated health practitioners before approval to allow COVID19 vaccination in pregnant women.
FAQ by @MoHFW_INDIA circulated among designated health practitioners before approval to allow COVID19 vaccination in pregnant women.https://twitter.com/malini_aisola/status/1405128195855159297





https://twitter.com/ShobhanSaxena/status/1410741584979644419

 Indian govt.
Indian govt.

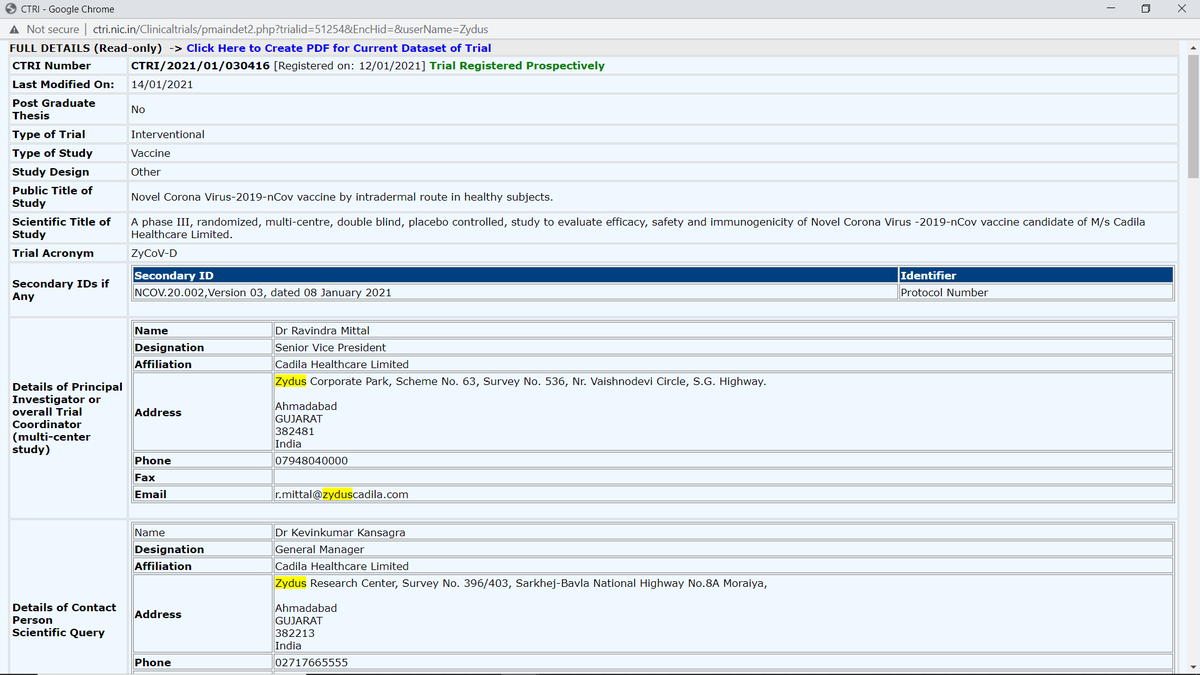
 2. Permission granted by DCGI on 2 Jun 2021 to initiate human trials adaptive Phases I & II.
2. Permission granted by DCGI on 2 Jun 2021 to initiate human trials adaptive Phases I & II.



 w/ minimum gap b/w 2 doses set to be 4 weeks.
w/ minimum gap b/w 2 doses set to be 4 weeks. 



 a) Thromobocytopenia is listed as common (≥1/100 to <1/10) adverse reaction
a) Thromobocytopenia is listed as common (≥1/100 to <1/10) adverse reaction

https://twitter.com/kakape/status/1372315648894844940Up to 28 Feb 2021:

https://twitter.com/das_seed/status/1346400481900421121

 👉Primary obj: efficacy of ZyCoV-D in prevention of virologically confirmed symptomatic COVID19 cases vs placebo (Day 70 to 364)
👉Primary obj: efficacy of ZyCoV-D in prevention of virologically confirmed symptomatic COVID19 cases vs placebo (Day 70 to 364)

https://twitter.com/malini_aisola/status/1350353133843611653
 - Total sessions (both types of sessions held, i.e., COVAXIN and COVISHIELD): 3351.
- Total sessions (both types of sessions held, i.e., COVAXIN and COVISHIELD): 3351. https://twitter.com/das_seed/status/1349040458853470209


https://twitter.com/AnooBhu/status/1309118055260323846


 Ashraya Hospital, Chikmagalur, Karnataka
Ashraya Hospital, Chikmagalur, Karnatakahttps://twitter.com/KirikKeerthi/status/1306815091283648512

https://twitter.com/kiranshaw/status/1282173158519222273

 To understand how HUGE scientific rationale can be, check her discussion w/ @SadhguruJV, a repeat offender. She speaks on DATA as well.
To understand how HUGE scientific rationale can be, check her discussion w/ @SadhguruJV, a repeat offender. She speaks on DATA as well. https://twitter.com/kiranshaw/status/1282299124209995776


https://twitter.com/das_seed/status/1278288047050829824@WHO's definition of community transmission allows govts to announce/deny community transmission based on their agenda. Maybe vague but @MoHFW_INDIA is NOT in position to defend its denial. "COMMUNITY TRANSMISSION" is officially used in @MoHFW_INDIA's critical policy/rules. \2


 ❗️Some MISINFORMATION based on outdated @WHO's surveillance guidelines & info (~March '20).
❗️Some MISINFORMATION based on outdated @WHO's surveillance guidelines & info (~March '20). https://twitter.com/das_seed/status/1268788580211712001\2

https://twitter.com/das_seed/status/1266655857556828161❗️PRE-SYMPTOMATIC ≠ ASYMPTOMATIC.
https://twitter.com/das_seed/status/1268686138602131459\2



 A/c to OL: Rate per Face Shield (FS) is INR 120/- before April 14. After April 14, the rate was reduced to INR 75/- for FS from other vendors. Changing the design and manufacturing process FS price was reduced to INR 60/-.
A/c to OL: Rate per Face Shield (FS) is INR 120/- before April 14. After April 14, the rate was reduced to INR 75/- for FS from other vendors. Changing the design and manufacturing process FS price was reduced to INR 60/-. 
