1/ We've mentioned before that extra utilization for DBD Hearts is 81% when using $TMDX.
The comparison between OCS and UNOS SRTR in the tables below is pretty powerful. Look at % in p. 36.
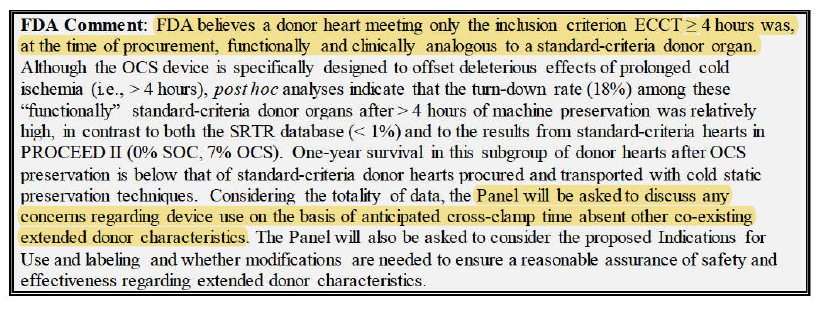
OCS can expand pool into donors w/ various risk factors (previously unused organs).

The comparison between OCS and UNOS SRTR in the tables below is pretty powerful. Look at % in p. 36.
OCS can expand pool into donors w/ various risk factors (previously unused organs).


2/ 19% of 93 hearts in study turned down.
Main reason being lactate rising, which is a biomarker mentioned by Dr. Schroder in presentation earlier.
Out of 75 hearts used...
24% age >65%
64% history of mechanical circ support
16% F-to-M mismatch
15% renal dysfunction
...


Main reason being lactate rising, which is a biomarker mentioned by Dr. Schroder in presentation earlier.
Out of 75 hearts used...
24% age >65%
64% history of mechanical circ support
16% F-to-M mismatch
15% renal dysfunction
...



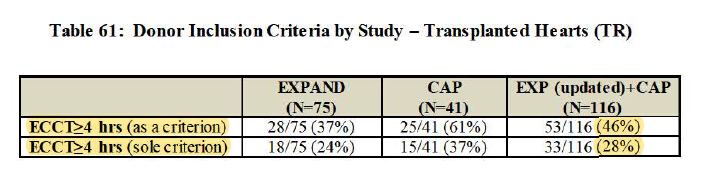
3/ 6.3hr cross-clamp (while w/ cold storage, 85% of hearts transplanted are <4hr, so major UPLIFT)
*Highest cross-clamp time in study of 11.4hr... wow
while minimizing cold ischemic time
Primary effectiveness (on 30-day survival and no severe ISHLT PGD): 88% vs. 65% perf goal

*Highest cross-clamp time in study of 11.4hr... wow
while minimizing cold ischemic time
Primary effectiveness (on 30-day survival and no severe ISHLT PGD): 88% vs. 65% perf goal


4/ From the study, can see only 10.7% in severe Primary Graft Dysfunction (first 24 hr.), lower than other studies.
Again, lots of recipients are already unhealthy to begin with, so overall survival trends down post-Tx. Should focus on cardiac survival made possible by $TMDX.



Again, lots of recipients are already unhealthy to begin with, so overall survival trends down post-Tx. Should focus on cardiac survival made possible by $TMDX.




5/ 4 deaths in EXPAND trial
(70/74 survived; 75th needed retransplant)
Death from defects in liver, lung, and even car accident
With EXPAND Cap, even higher survival than EXPAND at 100%. 2.4% of severe PGD.
Same trend goes for overall vs. cardiac survival in EXPAND + CAP.


(70/74 survived; 75th needed retransplant)
Death from defects in liver, lung, and even car accident
With EXPAND Cap, even higher survival than EXPAND at 100%. 2.4% of severe PGD.
Same trend goes for overall vs. cardiac survival in EXPAND + CAP.



P.S. Want to clarify that 24% in 2nd tweet in thread is RECIPIENT age >65 i.e. 18/75. If you look at screenshot, it’s clearly labeled.
For the trial, DONOR age >55 is 11.8% (11/93) per 1st message in thread.
Suggest you look at actual screenshots along with commentary.
For the trial, DONOR age >55 is 11.8% (11/93) per 1st message in thread.
Suggest you look at actual screenshots along with commentary.
Re: tweet 5 of the thread, 70/74 survived as of 30 DAYS POST-TRANSPLANT, which is the secondary endpoint measured.
In total trial timeframe, there are indeed more deaths:
PGD: 4
Multiorgan Failure: 2
Pneumonia: 1
Pulmonary Embolus: 1
Severe AB & Cellular Rejection: 1
In total trial timeframe, there are indeed more deaths:
PGD: 4
Multiorgan Failure: 2
Pneumonia: 1
Pulmonary Embolus: 1
Severe AB & Cellular Rejection: 1
The 4 acute severe PGD cases happened very soon after actual Transplant.
3 cases within 24hrs.
1 case within 48hrs.
Believe this means the rest of the deaths came from completely other sources and were after 30-day mark.
Again, Recipients were not the most healthy lot.
3 cases within 24hrs.
1 case within 48hrs.
Believe this means the rest of the deaths came from completely other sources and were after 30-day mark.
Again, Recipients were not the most healthy lot.

• • •
Missing some Tweet in this thread? You can try to
force a refresh