Encouraging studies! 🧵
An analysis of cross-reactive viral binding and neutralization of emerging SARS-CoV-2 variants shows Novavax’s and Moderna’s vaccines elicit immune responses that are effective against variants B.1.429 (CA) and B.1.351 (S. Africa).
nejm.org/doi/full/10.10…
An analysis of cross-reactive viral binding and neutralization of emerging SARS-CoV-2 variants shows Novavax’s and Moderna’s vaccines elicit immune responses that are effective against variants B.1.429 (CA) and B.1.351 (S. Africa).
nejm.org/doi/full/10.10…

Patients previously infected with SARS-CoV-2 received Pfizer’s vaccine. Before vaccination, they had neutralizing activity against variants B.1.1.7 & P.1 but not B.1.351. AFTER one dose, neutralizing activity against ALL variants increased substantially!
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…

Convalescent serum from those who recovered from an infection with SARS-CoV-2 variant B.1.351 showed potent neutralization of D614G (original), as well as variants B.1.351 (S. Africa) AND P.1 (Brazil). Booster vaccines may just seal the deal if needed!
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…

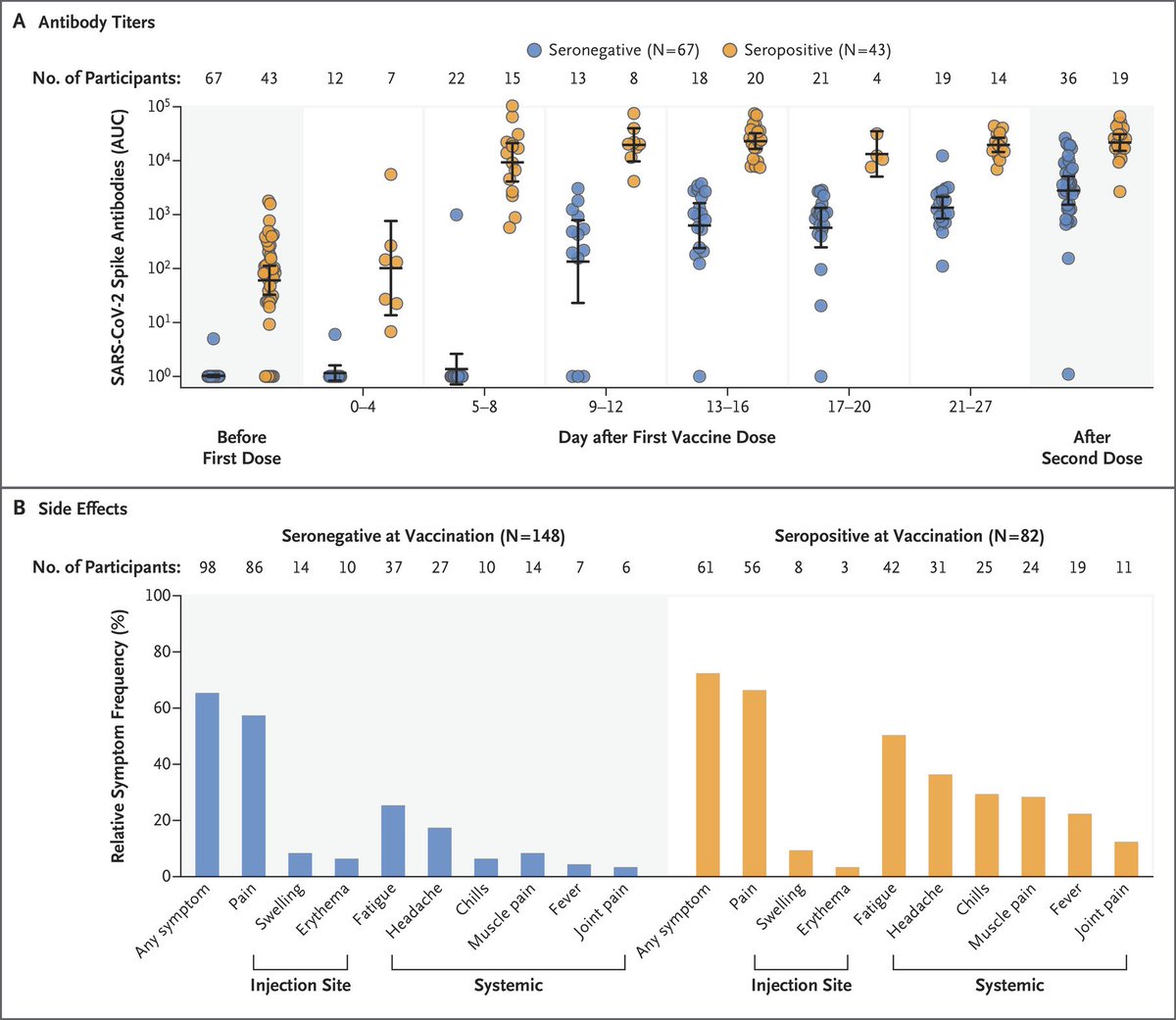
3 weeks after a single dose, those with recent SARS-CoV-2 infection or seropositive status had higher levels of antibody to 4 SARS-CoV-2 antigens and higher levels of antibodies with neutralizing characteristics than those without a history of infection.
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…

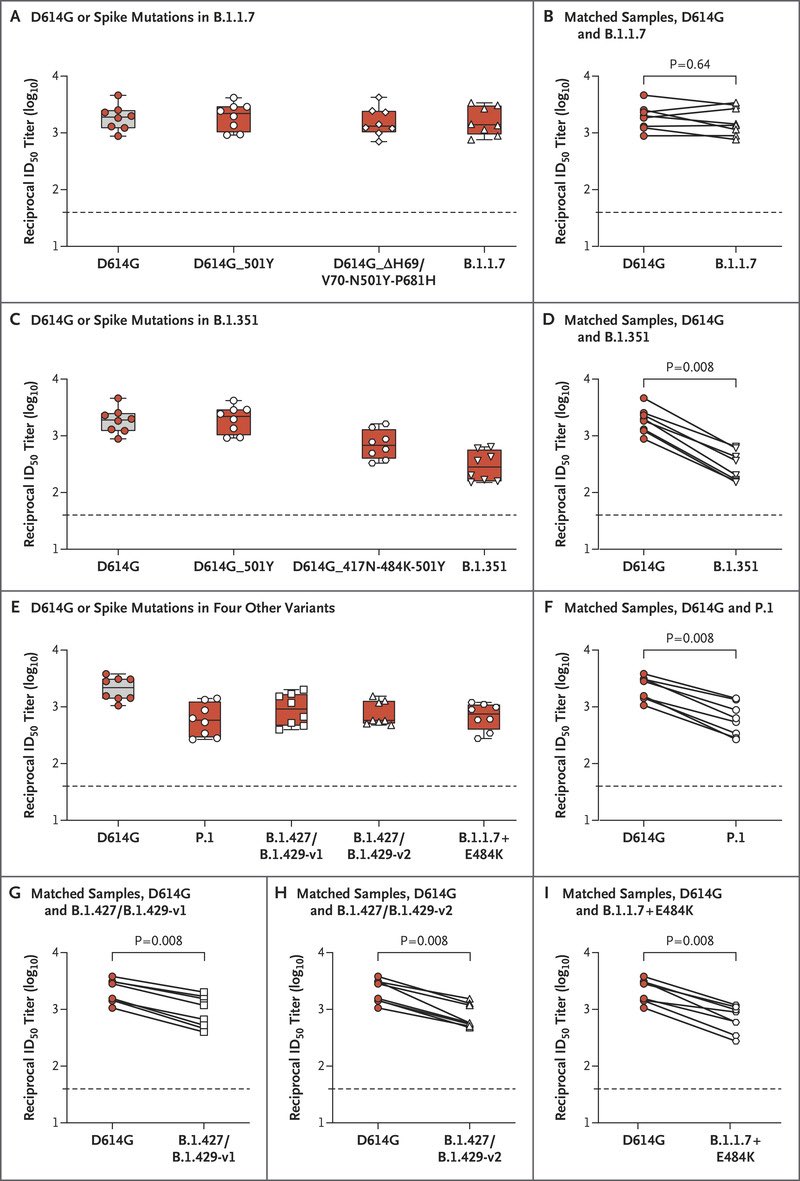
Moderna’s vaccine significantly reduces neutralizing activity against VOCs B.1.1.7 (UK), B.1.1.7+ E484K, P.1 (Brazil), B.1.351 (S. Africa) AND B.1.427/B.1.429 (CA).
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
Data from a UK clinical study suggests the AstraZenecas vaccine is 70.4% effective against symptomatic COVID-19 caused by variant B.1.1.7. The vaccine was 81.5% effective in preventing symptomatic COVID-19 caused by non-B.1.1.7 strains.
thelancet.com/journals/lance…
thelancet.com/journals/lance…

Antibody titers of vaccinees who recovered from a previous SARS-CoV-2 infection were found to be 10x to 45x as high as those of vaccinees without preexisting immunity at the same time points after just one dose of either mRNA vaccine.
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…
Serum samples obtained from participants vaccinated with two doses of Pfizer’s BNT162b2 COVID-19 vaccine shows effective neutralization against VOCs B.1.1.7 (UK), B.1.351 (S. Africa) AND P.1 (Brazil).
nejm.org/doi/full/10.10…
nejm.org/doi/full/10.10…

Why is this all good news? Because the vaccines are effective! This thread includes something on just about each of them. These also don’t even take into account cell-mediated immunity/ my love of T-cells which add a whole other layer of protection!
Have a great day! ☀️🧬🦠🧫💉
Have a great day! ☀️🧬🦠🧫💉
• • •
Missing some Tweet in this thread? You can try to
force a refresh








