Today at #AACR21 I was honored to present results of the phase 3 CheckMate 816 neoadjuvant chemo-nivo lung cancer trial on behalf of all the colleagues, patients & families who made it possible. In this thread I will discuss why we did the study, results and next steps/ 

This study built on an investigator-initated trial @ChaftJamie @SmithImmunology, @JulieBrahmer, @dpardol1 and I (many others have continued this work with larger phase 2 trials) reported back in 2018
nejm.org/doi/10.1056/NE…
nejm.org/doi/10.1056/NE…
In that small trial (supported by @AACR @SU2C & @LUNGevity among others) 20 pts had their lung cancers removed at surgery after 2 doses of nivo. 11 tumors underwent major pathological response, treatment was well tolerated and nivo appeared to drive an anti-tumor immune response.
Until recently there were no systemic therapy advances for surgical lung cancer for >10 yrs & the majority of pts w stage 2 or 3 lung cancer experience relapse of their cancer after surgery. Several phase 2 trials have shown chemo-IO to be active in this setting @MARIANOPROVENCI 

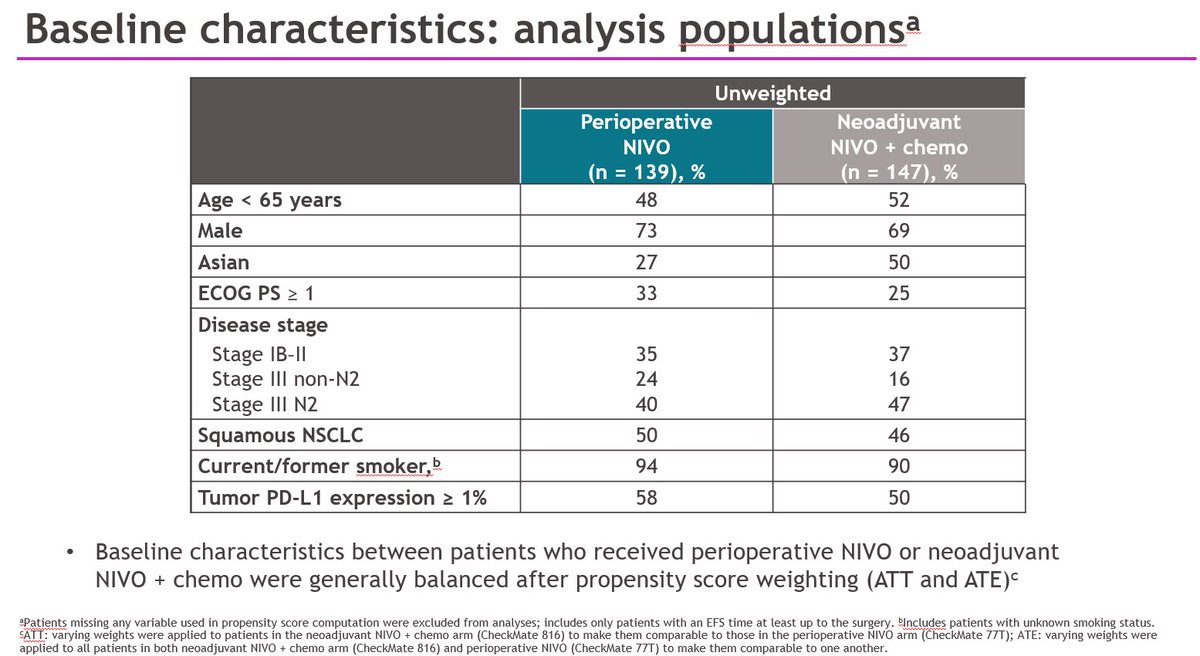
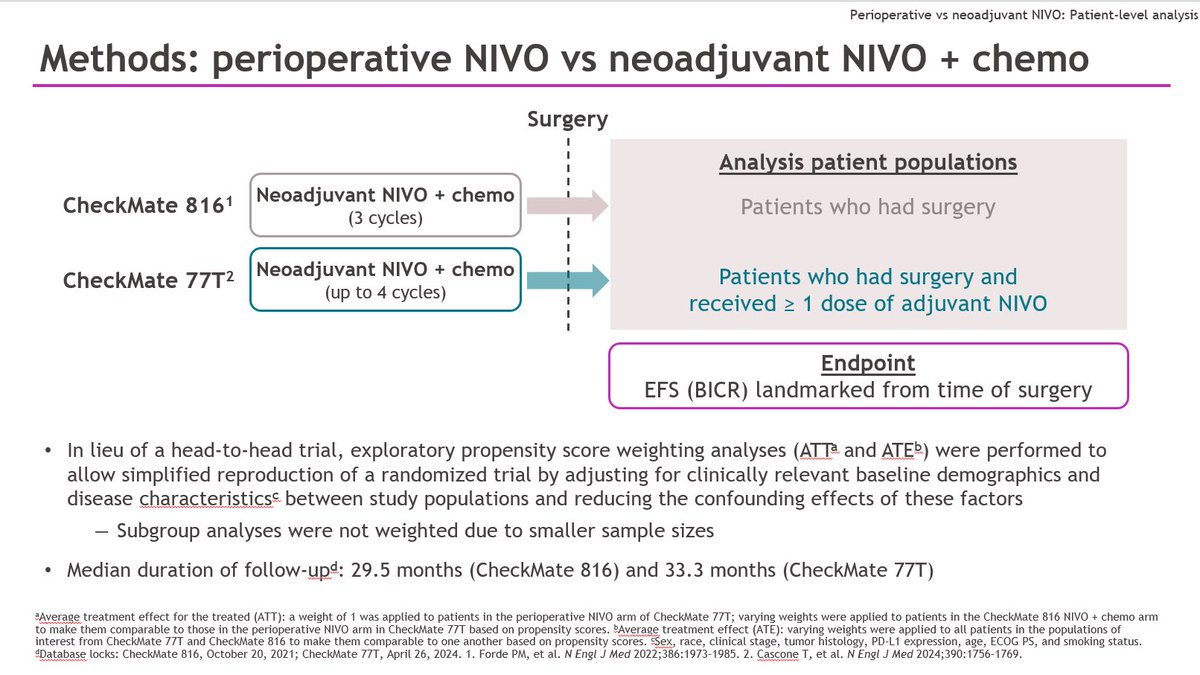
In Checkmate 816 we compared 3 cycles of neoadjuvant chemo-nivo to 3 cycles of chemo. The primary endpoints were pathological complete response (no remaining cancer in the primary or lymph nodes) & event-free survival. Today I reported the pCR results & correlates incl ctDNA 

83% (nivo chemo) vs. 75% (chemo alone) of pts had definitive surgery. More pts in the nivo- chemo arm were able to have lung sparing surgery. Delays to surgery past the predefined 6 weeks were mainly administrative (OR list space, scheduling around holidays etc) 

The addition of nivo to chemo increased pCR from 2.2% w chemo alone to 24% w chemo-nivo (p<0.0001). pCR was assessed by central pathologists who were blinded to trial arms. Surgical specimens had a median of 10% residual tumor cells after chemo-nivo vs 74% after chemo alone. 



There was no increase in grade 1-2 or grade 3-4 adverse events when neoadjuvant nivo was added to chemo and rates of immune mediated side effects were low, only 2 pts had pneumonitis and both were grade 1-2 

In a subset analysis (of 90 pts) rates of ctDNA clearance were higher during the preop phase with nivo-chemo and pCR rates were higher when ctDNA cleared 

Adding neoadjuvant nivo to chemo improved pCR, radiographic response & did not impair surgery. We await results for EFS & eventually OS however the data today are encouraging. My sincere gratitude to our pts, my colleagues, and mentors who brought this study to fruition. #lcsm 

• • •
Missing some Tweet in this thread? You can try to
force a refresh