A thread on recent updates (24 March- 9 April 2021) on Vaxzevria, a COVID19 vaccine mfg by AstraZeneca (called COVISHIELD if the same product is mfg by @SerumInstIndia).
1. Summary of productcharacteristics updated by @AstraZeneca after @EU_Commission's decision. Importantly,


1. Summary of productcharacteristics updated by @AstraZeneca after @EU_Commission's decision. Importantly,



a) Thromobocytopenia is listed as common (≥1/100 to <1/10) adverse reaction
b) Thrombosis in combination w/ thrombocytopenia is listed as very rare (<1/10,000) adverse reaction
c) Currently available trial data do not allow an estimate of vaccine efficacy in 55+ yrs age group.
b) Thrombosis in combination w/ thrombocytopenia is listed as very rare (<1/10,000) adverse reaction
c) Currently available trial data do not allow an estimate of vaccine efficacy in 55+ yrs age group.

d) Asks healthcare professionals to be on alert for signs & symptoms of thromboembolism &/or thrombocytopenia. Instructs vaccinated people to seek immediate medical attention if they develop symptoms such as shortness of breath, chest pain, leg swelling, persistent abdominal pain 



following vaccination. Additionally, anyone w/ neurological symptoms including severe or persistent headaches or blurred vision after vaccination, or who experiences skin bruising (petechia) beyond the site of vaccination after a few days, should seek prompt medical attention.
2. Package leaflet updated by @AstraZeneca. Some important mentions are:
a) Mentions that 2-dose vaccination course of Vaxzevria may not fully protect all those who
receive it. Not known how long protection lasts. Currently there are limited data on efficacy of Vaxzevria in 55+

a) Mentions that 2-dose vaccination course of Vaxzevria may not fully protect all those who
receive it. Not known how long protection lasts. Currently there are limited data on efficacy of Vaxzevria in 55+


yrs age group.
b) In possible side-effects,
i) asks to get URGENT medical attention if recipient gets symptoms of a severe allergic reaction. Such reactions may include a combination of any of following symptoms: feeling faint or light-headed, changes in heartbeat, shortness of
b) In possible side-effects,
i) asks to get URGENT medical attention if recipient gets symptoms of a severe allergic reaction. Such reactions may include a combination of any of following symptoms: feeling faint or light-headed, changes in heartbeat, shortness of

breath, wheezing, swelling of lips, face, or throat, hives or rash, nausea or vomiting, stomach pain.
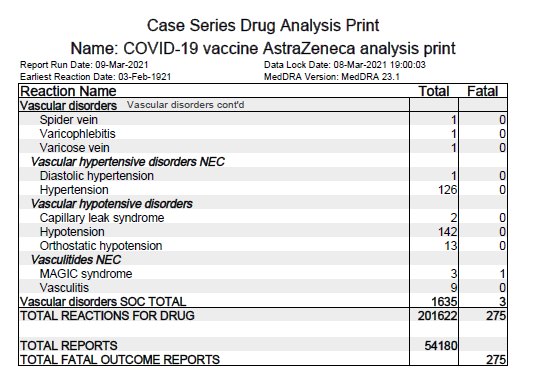
ii) Low level of blood platelets listed as common AEFI (may affect up to 1 in 10 people)
iii) Blood clots often in unusual locations (e.g. brain, bowel, liver, spleen) in
ii) Low level of blood platelets listed as common AEFI (may affect up to 1 in 10 people)
iii) Blood clots often in unusual locations (e.g. brain, bowel, liver, spleen) in

combination w/ low level of blood platelets listed as very rare AEFI (may affect up to 1 in 10000 people).
3. @AstraZeneca in agreement w/ EMA & <National Competent Authority> released alert for health professionals on risk of thrombocytopenia & coagulation disorders, 24/03/2021

3. @AstraZeneca in agreement w/ EMA & <National Competent Authority> released alert for health professionals on risk of thrombocytopenia & coagulation disorders, 24/03/2021


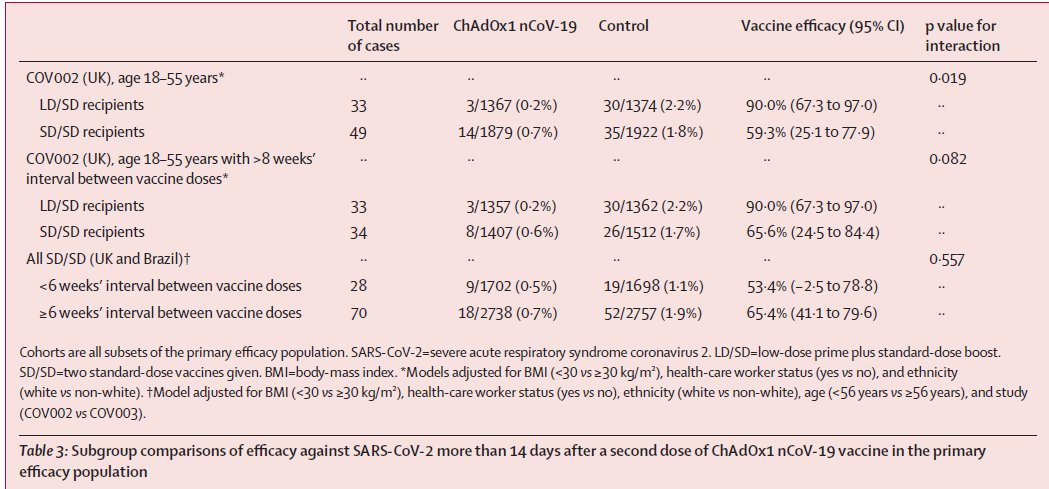
4. PRAC, EMA has started the review of a safety signal to assess AEFI reports of Capillary Leak Syndrome (CLS) (after 5 CLS cases were reported).
For CLS: en.wikipedia.org/wiki/Capillary….
References: ema.europa.eu/en/medicines/h…; gov.uk/government/pub…


For CLS: en.wikipedia.org/wiki/Capillary….
References: ema.europa.eu/en/medicines/h…; gov.uk/government/pub…
https://twitter.com/das_seed/status/1380886691045576704

A short cautionary note on mgmt of patients suffering from COVID-19 Vaccine induced Thrombosis & Thrombocytopenia (VITT).
1. Standard treatments for blood clots on VITT patients can actually cause tremendous harm or outcome can be fatal. @US_FDA
1. Standard treatments for blood clots on VITT patients can actually cause tremendous harm or outcome can be fatal. @US_FDA
https://twitter.com/das_seed/status/1382417163282173960
2. Guidance on COVID-19 Vaccine induced Thrombosis & Thrombocytopenia (VITT) by Expert Haematology Panel, UK, 7 Apr '21: b-s-h.org.uk/media/19530/gu…
3. Interim Guidelines on Vaccine-Induced Prothrombotic Immune Thrombocytopenia (VIPIT) by UN, 12 Apr '21: un.org/sites/un2.un.o…


3. Interim Guidelines on Vaccine-Induced Prothrombotic Immune Thrombocytopenia (VIPIT) by UN, 12 Apr '21: un.org/sites/un2.un.o…



4. Medical case studies on Thrombosis w/ Thrombocytopenia Syndrome (TTS) (also VIPIT or VITT) post COVISHIELD
- Austria & Germany: nejm.org/doi/full/10.10… DOI: 10.1056/NEJMoa2104840 (9 April 2021)
- Norway: nejm.org/doi/full/10.10… DOI: 10.1056/NEJMoa2104882 (9 April 2021)

- Austria & Germany: nejm.org/doi/full/10.10… DOI: 10.1056/NEJMoa2104840 (9 April 2021)
- Norway: nejm.org/doi/full/10.10… DOI: 10.1056/NEJMoa2104882 (9 April 2021)


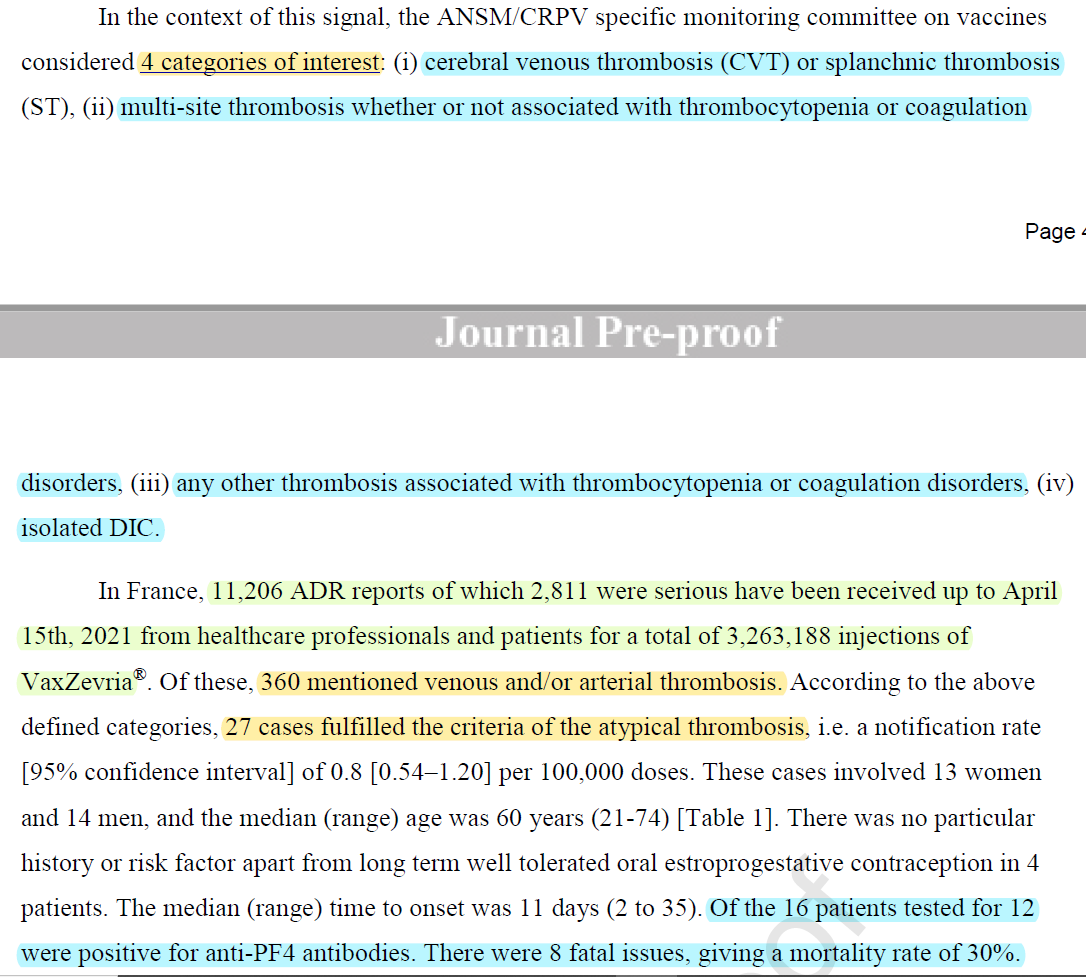
🚨 Atypical thrombosis associated w/ VaxZevria (COVISHIELD)
- France: doi.org/10.1016/j.ther…
In some cases w/ similar clinical/radiological pictures as TTS, anti-PF4 antibodies remained -ve & in some of those cases no thrombocytopenia or coagulation disorders were observed.


- France: doi.org/10.1016/j.ther…
In some cases w/ similar clinical/radiological pictures as TTS, anti-PF4 antibodies remained -ve & in some of those cases no thrombocytopenia or coagulation disorders were observed.


🚨Product info of Vaxzevria (COVISHIELD), approved by CHMP on 20 May 2021. Some revisions on possible AEFIs:
Addition of Immune system disorders (unknown frequency)– Anaphylaxis & Hypersensitivity.
Mentions that transient mild thrombocytopenia was commonly reported in trials.


Addition of Immune system disorders (unknown frequency)– Anaphylaxis & Hypersensitivity.
Mentions that transient mild thrombocytopenia was commonly reported in trials.



Contraindications to not give booster dose of COVISHIELD to those who previously had TTS after COVISHIELD.
Those w/ thrombocytopenia w/in 3 week after COVISHIELD be alert of thrombosis, & vice-versa.
Hypersensitivity reactions presenting as hives or rapid swelling under skin in

Those w/ thrombocytopenia w/in 3 week after COVISHIELD be alert of thrombosis, & vice-versa.
Hypersensitivity reactions presenting as hives or rapid swelling under skin in


in areas such as face, lips, mouth & throat are newly identified side effects. [Hypersensitivity reaction of urticaria as new uncommon side effect, & angioedema]
AEFIs cases of Capillary leak syndrome, Guillain-Barré syndrome, & Acute macular neuroretinopathy are under review.


AEFIs cases of Capillary leak syndrome, Guillain-Barré syndrome, & Acute macular neuroretinopathy are under review.



• • •
Missing some Tweet in this thread? You can try to
force a refresh