
Anvisa (Brazil's drug regulator) has just announced it has denied the request to import Sputnik V vaccine because of absence of data & issues with the development of the vaccine, including vax quality. g1.globo.com/bemestar/vacin… Meeting still streaming ..1/n
...Unanswered questions about the vaccine include basic biological data about its actions in the body, data on adverse events (including questions about thromboembolic events) ...2/n 

...I think with all the focus on Lancet paper for phase 3, people forget how little study there had been of this vax: eg only 38 people in phase 1/2 trial version with lots of problems. (absolutelymaybe.plos.org/2020/09/11/pha…) Assessing a vaccine isn't only about the phase 3 trial results...3/n 

...They pointed to issues with reporting of adverse events in the phase 3 trial (I wrote about this here: absolutelymaybe.plos.org/2021/03/16/com…). Remember: deficiencies in a trial/study program are not the same as deficiencies in a vax, but they affect how certain we can be about the vax...4/n 
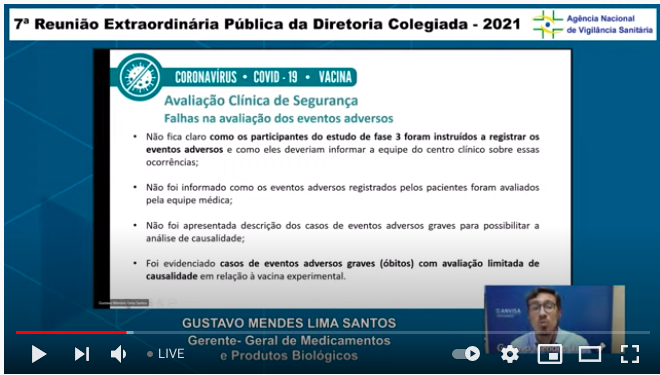
..*So* wishing I understood Portuguese properly right now! Big thanx to @gutoaqui who's translating these slides as I post them.
Here pointing to uncertainties around vax efficacy because of biases that could arise from the way they ascertained & determined cases of Covid-19..5/n
Here pointing to uncertainties around vax efficacy because of biases that could arise from the way they ascertained & determined cases of Covid-19..5/n
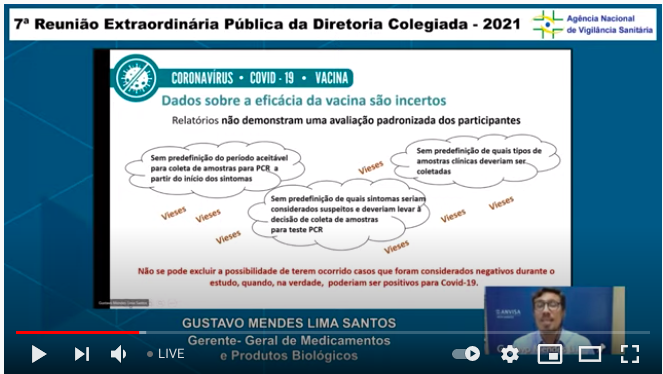
... This is depressing. Basically, the developers have failed to provide enough data to back up their claims of efficacy & have left a string of open questions about te vaccine's development. ...6/n 

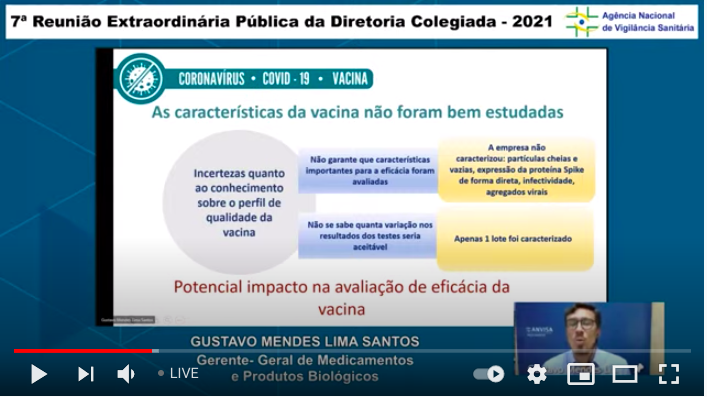

...Questions about quality control of manufacturing. (I'm now at the point I'm glad I don't understand enough Portuguese - this is too depressing) ...7/n 

...Distilling some of the fundamental issues lacking here: assurance of potency of the formulation (if I understand that correctly) & basic issues about how replication for the adenoviral vectors in both shots...8/n 
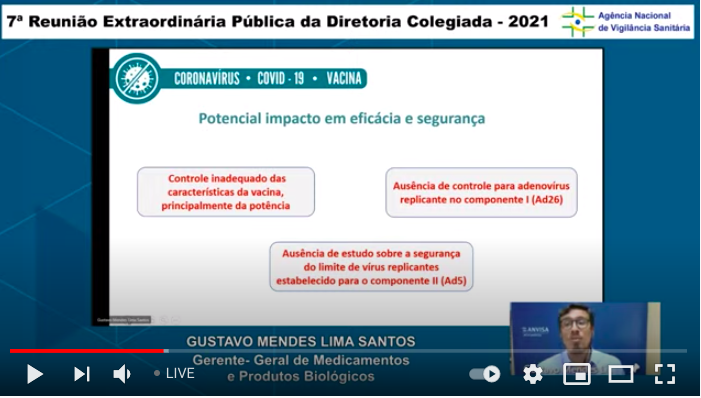
...The catalog of what's missing to explain the action of these vaccines continues: here basics about spike protein & replication of the Ad26 component (the 1st dose of Sputnik V). (I'm only including some of the slides, not all of them) ...9/n 

...Onto trying to establish the credentials of the manufacturing sites: couldn't, if I'm understanding this correctly.
I've seen enough. This seems very thorough work again from Anvisa. For those who want to see more, a reminder it's here 10/10
I've seen enough. This seems very thorough work again from Anvisa. For those who want to see more, a reminder it's here 10/10

@gutoaqui Thank you so much!
PS: The adenovirus in Covid vax is not supposed to replicate (it's supposed to be just a carrier). Specific critical detail on Anvisa's Sputnik V evaluation I missed: replicating adenovirus was identified in every lot of the vax inspected coronavirus.atarde.com.br/diretoria-da-a… HT @swiftshoes
Important PPS: the Ad26 vax lots (first Sputnik V shot) not yet analyzed - this is what was identified in every lot of the Ad5 vax (2nd shot) HT @Dereklowe
https://twitter.com/DavidRach2/status/1387183627193229313
• • •
Missing some Tweet in this thread? You can try to
force a refresh







