A short note on dosing regimen of COVISHIELD for complete vaccination.
We've 3 different set of trials:
1. UK/Brazil trials
This trial was open-label, w/ scandalous error in dosing regimen ("half-dose" error), had multiple major amendments in protocol when trial was undergoing
We've 3 different set of trials:
1. UK/Brazil trials
This trial was open-label, w/ scandalous error in dosing regimen ("half-dose" error), had multiple major amendments in protocol when trial was undergoing

w/ minimum gap b/w 2 doses set to be 4 weeks.
COVISHIELD was initially supposed to be a single dose vaccine, but in middle of trial they realized that booster dose was required for higher efficacy compared to just single dose.
In the process, due to mfg/supply delay, intended
COVISHIELD was initially supposed to be a single dose vaccine, but in middle of trial they realized that booster dose was required for higher efficacy compared to just single dose.
In the process, due to mfg/supply delay, intended

interval of 4 weeks was not achieved & got prolonged.
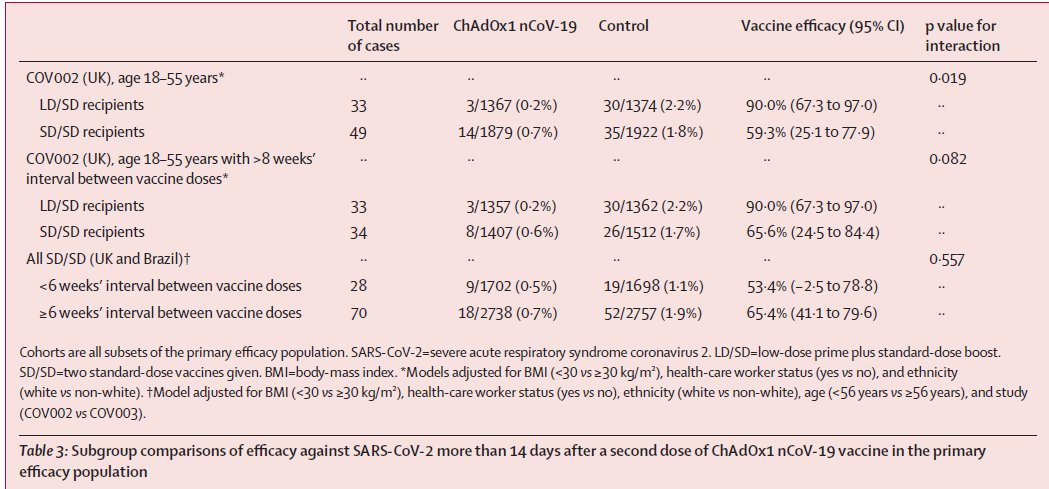
Attached are intended & extrapolated sub-group analysis that was carried out by PIs as part of protocol, w/ different dosing levels & intervals.
*extrapolated analyses were not part of protocol hypothesis but in response to

Attached are intended & extrapolated sub-group analysis that was carried out by PIs as part of protocol, w/ different dosing levels & intervals.
*extrapolated analyses were not part of protocol hypothesis but in response to

the editors/referees.
Extrapolatory analysis can often be statistical gymnastics where you beat the data to extent that it gives inferences confirming your bias.
No one bought half & full dose combo despite it showing better efficacy since it was not part of actual hypothesis.

Extrapolatory analysis can often be statistical gymnastics where you beat the data to extent that it gives inferences confirming your bias.
No one bought half & full dose combo despite it showing better efficacy since it was not part of actual hypothesis.


The median interval between doses for the SD/SD group in COV002 was 69 days, i.e., 9.85 weeks (CI: 50–86, i.e, approx b/w 7 weeks to 12 weeks). Conversely, the majority of participants in COV003 in the SD/SD group (2493 (61·0%) of 4088) received a 2nd dose w/in 6 weeks of 1st. 

Efficacy estimates from EMA, EU Commission for assessment of COVISHIELD was done with standard dosing regimen w/ 4 to 12 weeks as interval b/w 2 full doses.
2. India trial: This was bridging studies for safety & immunogenicity among Indian population

2. India trial: This was bridging studies for safety & immunogenicity among Indian population
https://twitter.com/das_seed/status/1393017922046636035

and to compare equivalence of COVISHIELD w/ Vaxzevria.
Condition for regulatory authorization/approval is based on efficacy analysis from large RCTs abroad, while also taking consideration of safety signals from these trials.
Dosing schedule for Indian trial was two full doses
Condition for regulatory authorization/approval is based on efficacy analysis from large RCTs abroad, while also taking consideration of safety signals from these trials.
Dosing schedule for Indian trial was two full doses
w/ interval of 4 weeks.
However, immunogenicity analysis was done at prematurely (against description of protocol). Even safety analysis have issues. Timeline & details of some important events regarding this are mentioned in thread attached.


However, immunogenicity analysis was done at prematurely (against description of protocol). Even safety analysis have issues. Timeline & details of some important events regarding this are mentioned in thread attached.
https://twitter.com/das_seed/status/1349064032276393987


3. US trial: Double-blind, large RCT w/ two standard doses at gap of fixed 4 weeks (28 days).
Trial results announced via press release was 76% efficacy against symptomatic COVID19 disease. It's more than efficacy from UK/Brazil trial for 2 std doses.


Trial results announced via press release was 76% efficacy against symptomatic COVID19 disease. It's more than efficacy from UK/Brazil trial for 2 std doses.
https://twitter.com/das_seed/status/1374921354550652934


It's CRITICAL to note that proper verification on efficacy of a vaccine initially comes from a well-blinded sufficiently large-sized randomized control trials. Real-world data acts as supporting evidences.
Real-world data have limitations because there can be number of factors
Real-world data have limitations because there can be number of factors
that could have major role compared to vaccine in outcomes we see from real-world data. [RECOVERY trials are great example to know why RCTs are important than assertions based on real-world data].
Now we can turn around discussion to current dosing interval in India.
Now we can turn around discussion to current dosing interval in India.
We have revised interval (gap) b/w 2 doses of COVISHIELD to 12 to 16 weeks based on extrapolatory sub-group analysis of UK/Brazil trial data, results published online on 8 December 2020 (last year).
Pics of recommendation from @WHO during Feb '21.


Pics of recommendation from @WHO during Feb '21.
https://twitter.com/das_seed/status/1393046587300057088


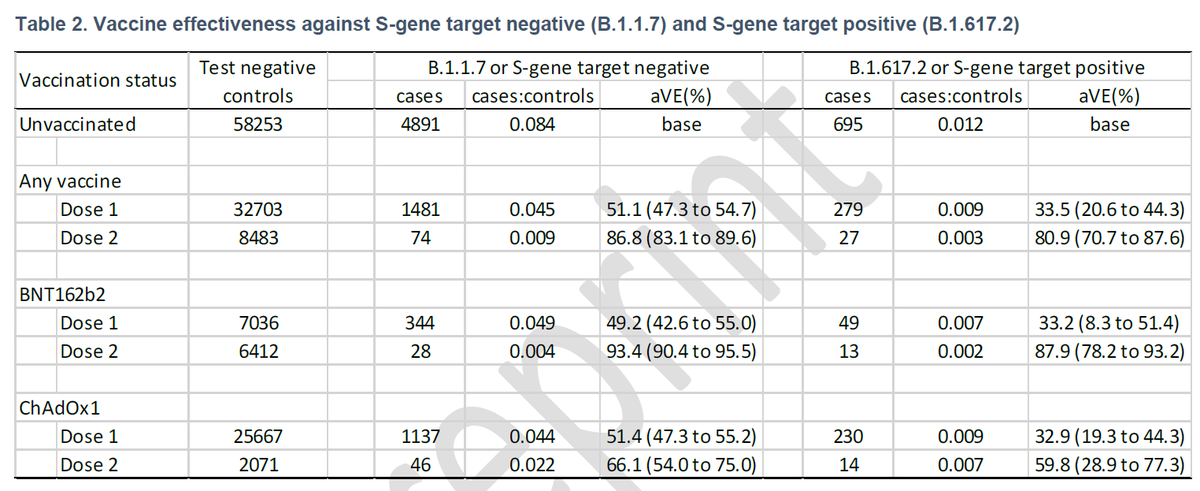
There's no proper real-world data from UK/India/EEA for dosing interval greater than 12 weeks (12-16 weeks) yet, because authorizations were w/ gap between 4 to 12 weeks in these nations. Only India extended the gap to 12 to 16 weeks, while UK reduced the gap to 4 to 8 weeks for
priority groups (50+ years and clinically vulnerable) amid real-world data showing concerning decrease in effectiveness against B.1.167.2 strains, 1st identified in India.
India will have to collect it's own real-world data for 12 to 16 weeks to perform

India will have to collect it's own real-world data for 12 to 16 weeks to perform
https://twitter.com/das_seed/status/1396397223731204097
statistical gymnastics.
COVID19 vaccines does provide some level of protection. What NTAGI COVID19 vaccine committee & NEGVAC should do is to prioritize two doses at interval of minimum 4 weeks for priority group (based on risk factor: age, clinical, occupation) so that they get
COVID19 vaccines does provide some level of protection. What NTAGI COVID19 vaccine committee & NEGVAC should do is to prioritize two doses at interval of minimum 4 weeks for priority group (based on risk factor: age, clinical, occupation) so that they get
better protection than just from single dose (which seems to be too low against B.1.167.2 strains).
For non-vulnerable group w/ comparatively low risk factors, it would be better if all these vaccines are for now diverted to those w/ high risks.
NEGAV should consider
For non-vulnerable group w/ comparatively low risk factors, it would be better if all these vaccines are for now diverted to those w/ high risks.
NEGAV should consider
differential dosing intervals based on risk/vulnerability of group.
There needs to be complete transparency over assessment & rationale behind decisions. We cannot hype or underplay efficacy & safety factors of vaccines. Need balance w/ facts.
There needs to be complete transparency over assessment & rationale behind decisions. We cannot hype or underplay efficacy & safety factors of vaccines. Need balance w/ facts.
https://twitter.com/das_seed/status/1353321815511334914
• • •
Missing some Tweet in this thread? You can try to
force a refresh