We are unveiling new findings about MME/day calcs for #opioids @US_FDA Workshop today. Live blogged in thread below.
Lab notebook, code, equations:
opioiddata.org/studies/equati…
With @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey
#epitwitter #RxEpi #medtwitter
Lab notebook, code, equations:
opioiddata.org/studies/equati…
With @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey
#epitwitter #RxEpi #medtwitter

@US_FDA @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey Apologies in advance... this is experimental live blogging... sometimes twitter scrambles images on long threads
@US_FDA @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey 14 states impose limits on opioid dosage that can be prescribed, ranging from 30 MME to a 120 MME daily maximum. Also third-party payers (insurance, @CMSgov). Analysis by @coreysdavis @amyamnesia of @networkforphl
onlinelibrary.wiley.com/doi/10.1111/ad…
onlinelibrary.wiley.com/doi/10.1111/ad…

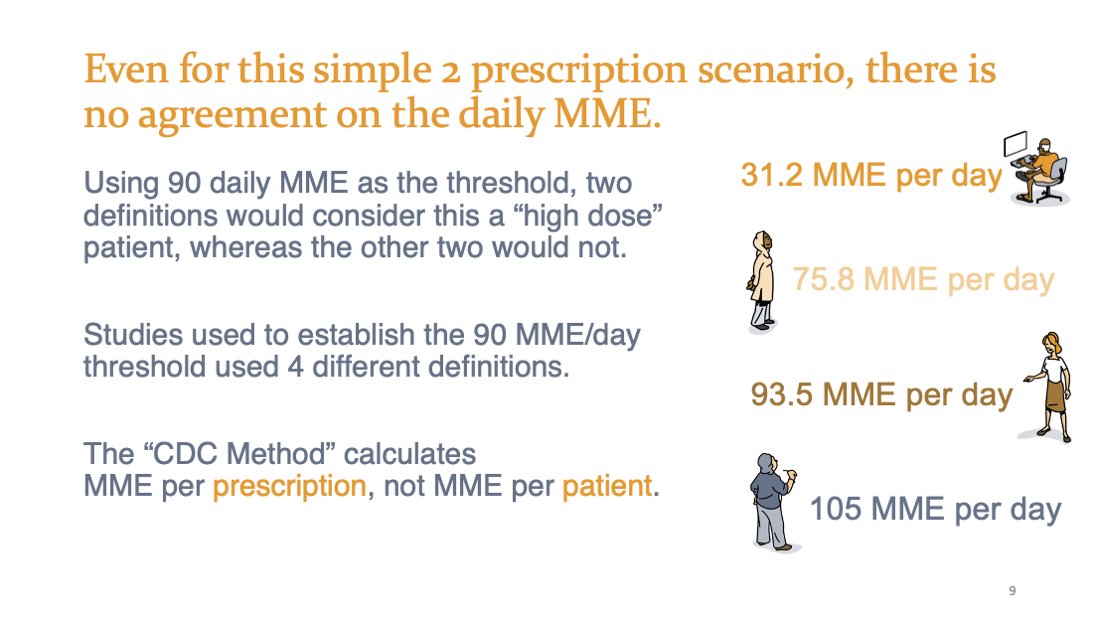
@US_FDA @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey @CMSGov @coreysdavis @amyamnesia @networkforphl Is this a "high dose" patient?
Rx1: 30 mg BID oxycodone ER, 30 days (#60) = 2,700mg
Rx2: 5mg oxycodone IR ~BID prn for first 7 days (#14) = 105mg
assume 1.5x conversion
Total: 2,805mg
Would you believe that some definitions say YES, and some definitions say NO? Srsly. 👇
Rx1: 30 mg BID oxycodone ER, 30 days (#60) = 2,700mg
Rx2: 5mg oxycodone IR ~BID prn for first 7 days (#14) = 105mg
assume 1.5x conversion
Total: 2,805mg
Would you believe that some definitions say YES, and some definitions say NO? Srsly. 👇

@US_FDA @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey @CMSGov @coreysdavis @amyamnesia @networkforphl Here's our revelation from reviewing the evidence base cited in the 2016 CDC Guideline: There are FOUR (4) different ways to calculate daily MME, not even considering conversion tables. The scenario above would yield:
31.2 MME/day
75.8 MME/day
93.5 MME/day
105 MME/day
31.2 MME/day
75.8 MME/day
93.5 MME/day
105 MME/day
@US_FDA @chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey @CMSGov @coreysdavis @amyamnesia @networkforphl Here's a detailed breakdown of methods limitations in the @CDCgov Guideline evidence base by @AnnaEAustin & other epidemiologists @UNCpublichealth
onlinelibrary.wiley.com/doi/full/10.10…
onlinelibrary.wiley.com/doi/full/10.10…
How do they differ? The denominator. It is the "day" part of "MME per day" that's been ignored.
How do you measure, measure a day in the life of a patient?
How do you measure, measure a day in the life of a patient?
Definition 1/4: Total Days Supply
Denominator is sum of days supply
2805mg/37days = 75.8 MME/day
Example: pubmed.ncbi.nlm.nih.gov/18574361/
Denominator is sum of days supply
2805mg/37days = 75.8 MME/day
Example: pubmed.ncbi.nlm.nih.gov/18574361/

Definition 2/4: On-therapy Days
Denominator is calendar days, counting overlap days once
2805mg/30days = 93.5 MME/day
Example: ncbi.nlm.nih.gov/pmc/articles/P…
Denominator is calendar days, counting overlap days once
2805mg/30days = 93.5 MME/day
Example: ncbi.nlm.nih.gov/pmc/articles/P…

Definition 3/4: Fixed Observation Window
Denominator is fixed at 90days for all patients
2805mg/90days = 31.2 MME/day
Example: ncbi.nlm.nih.gov/pmc/articles/P…
Denominator is fixed at 90days for all patients
2805mg/90days = 31.2 MME/day
Example: ncbi.nlm.nih.gov/pmc/articles/P…

Definition 4/4: Maximum Daily Dose
The "CDC Method" ignores dates and days supply for max single-day exposure, used in the CDC Opioid Guideline mobile app.
max = 90mg+15mg = 105 MME/day
Example: academic.oup.com/painmedicine/a…
The "CDC Method" ignores dates and days supply for max single-day exposure, used in the CDC Opioid Guideline mobile app.
max = 90mg+15mg = 105 MME/day
Example: academic.oup.com/painmedicine/a…

For reals, does any of this really matter?
Stay tuned: Subtle choices make huge differences.
Stay tuned: Subtle choices make huge differences.
A Controlled Experiment that mimics an observational policy/intervention evaluation.
Compare 2 places to see which has more "high dose" patients.
Same dataset, same conversion factors.
The ONLY source of variation comes from the4 definitions ofdaily MME.
Compare 2 places to see which has more "high dose" patients.
Same dataset, same conversion factors.
The ONLY source of variation comes from the4 definitions ofdaily MME.

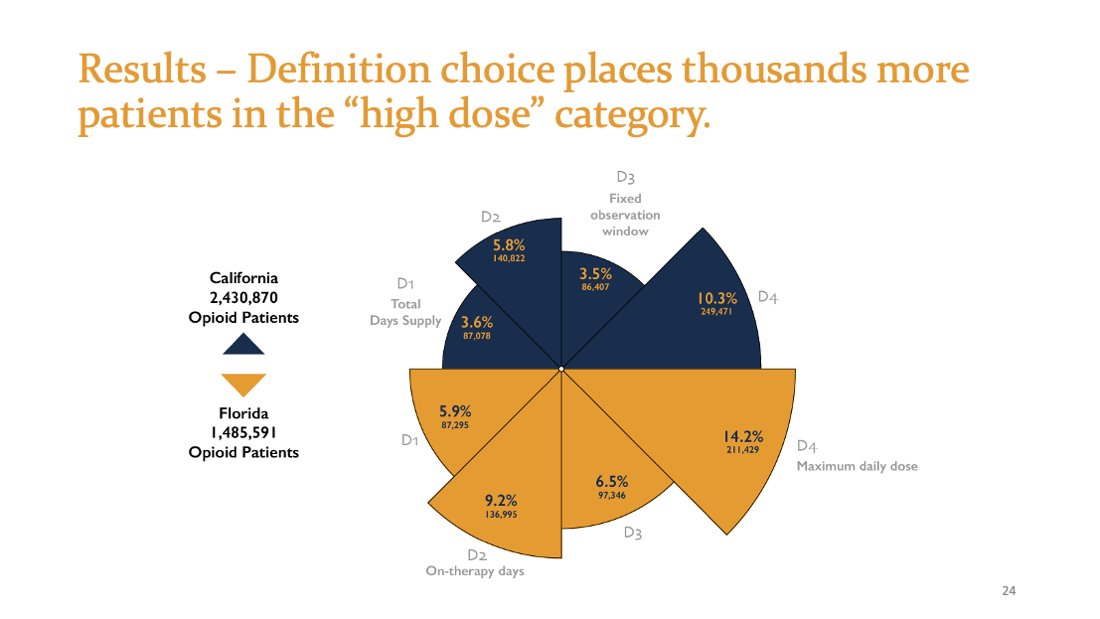
Methods. PDMP data for 3Q2018.
9,436,640 opioid analgesic prescriptions
California n=5,677,277
Florida n=3,759,363
3,916,461 unique adult residents
California n=2,430,870 (7.9 per 100 residents)
Florida n=1,485,591 (8.7 per 100 residents)
9,436,640 opioid analgesic prescriptions
California n=5,677,277
Florida n=3,759,363
3,916,461 unique adult residents
California n=2,430,870 (7.9 per 100 residents)
Florida n=1,485,591 (8.7 per 100 residents)

3-fold difference in which patients are considered high dose between D1 and D4. Both are described in published papers as the "CDC Method": 5.9% vs. 14.2% (FL)
Arbitrary definition choice impacts hundreds of thousands of patients with painful conditions.
Arbitrary definition choice impacts hundreds of thousands of patients with painful conditions.
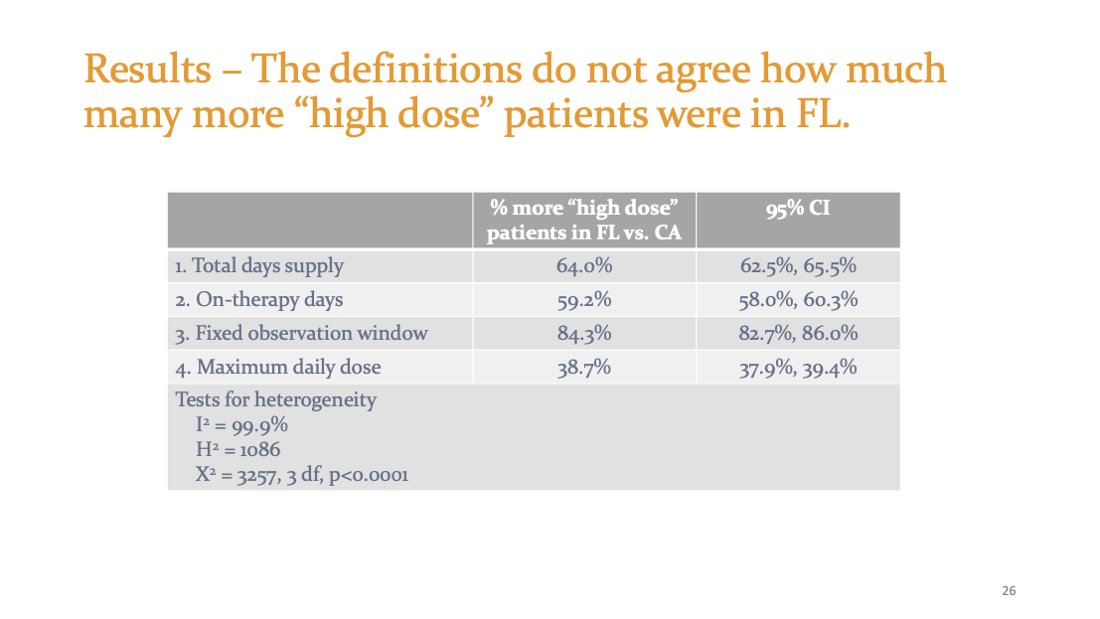
4 policy/intervention evaluations on the SAME DATA with the SAME CONVERSION TABLES wouldn't agree how many more "high dose" patients were in FL: 64%, 59%, 84%, or 39% more 
Any of these means or medians could have been justified scientifically. SAME DATA but 8 different results. See? Subtle choices have huge consequences. 
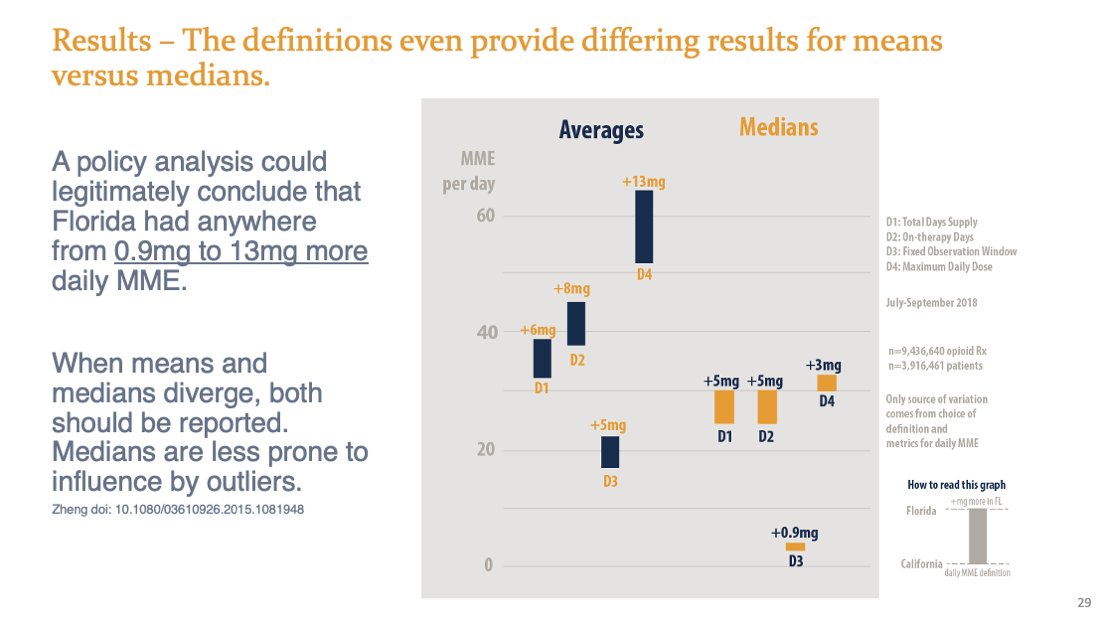
Would we say that doses are similar (1 mg difference) but lots more "high dose" patients in FL?
or
FL has a bit more "high dose" patients, but they are getting A LOT more (13 mg)?
Imma a say it again: Subtle choices have huge consequences in what we infer.
or
FL has a bit more "high dose" patients, but they are getting A LOT more (13 mg)?
Imma a say it again: Subtle choices have huge consequences in what we infer.

Why is this happening? Overlapping prescription days supply has a lot to do with it.
Epi/stats folks, beware. Changes in US prescribing patterns mean that certain definitions (D1) will attenuate intervention effects over time!

Epi/stats folks, beware. Changes in US prescribing patterns mean that certain definitions (D1) will attenuate intervention effects over time!


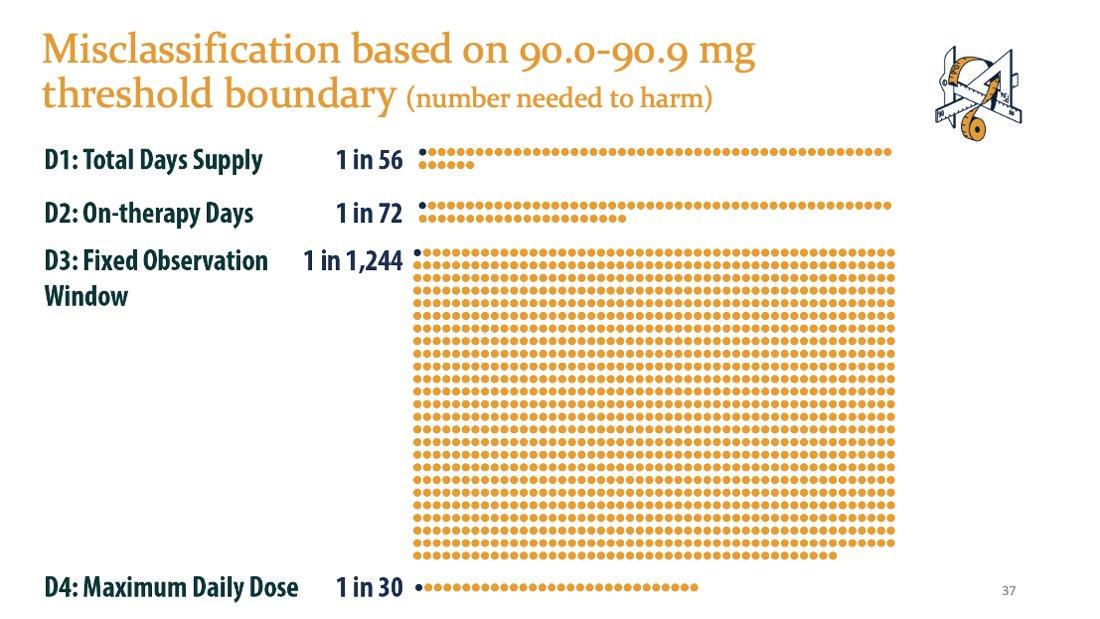
If you shift the “high dose” threshold from 90.9 to 90.0, you increase the number of “high dose” patients by 15.4% (CI: 15.2%, 15.7%). 

If an insurance company and physician use 90.0 vs. 90.9, they would disagree on "high dose" status in 1 out of every 30 patients with D4. 
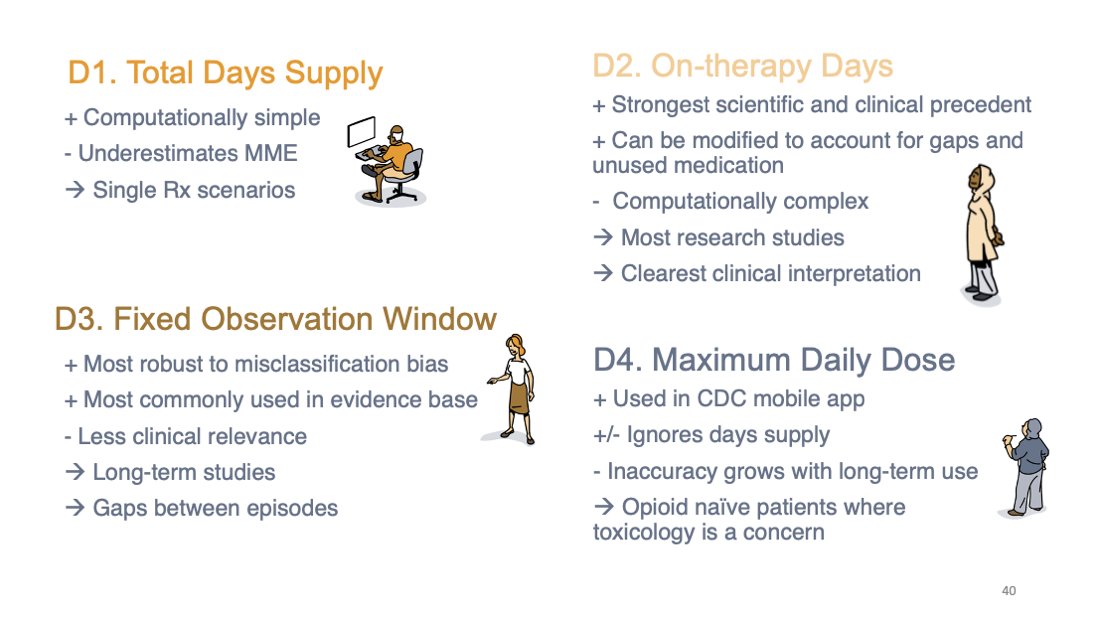
Which definition to use? I like D2 in general, but D3 is good for long-term use because it is more robust to misclassification. Do not use D1. If toxicity is short-term in opioid naive patients, D4 could possibly maybe be sorta acceptable. 
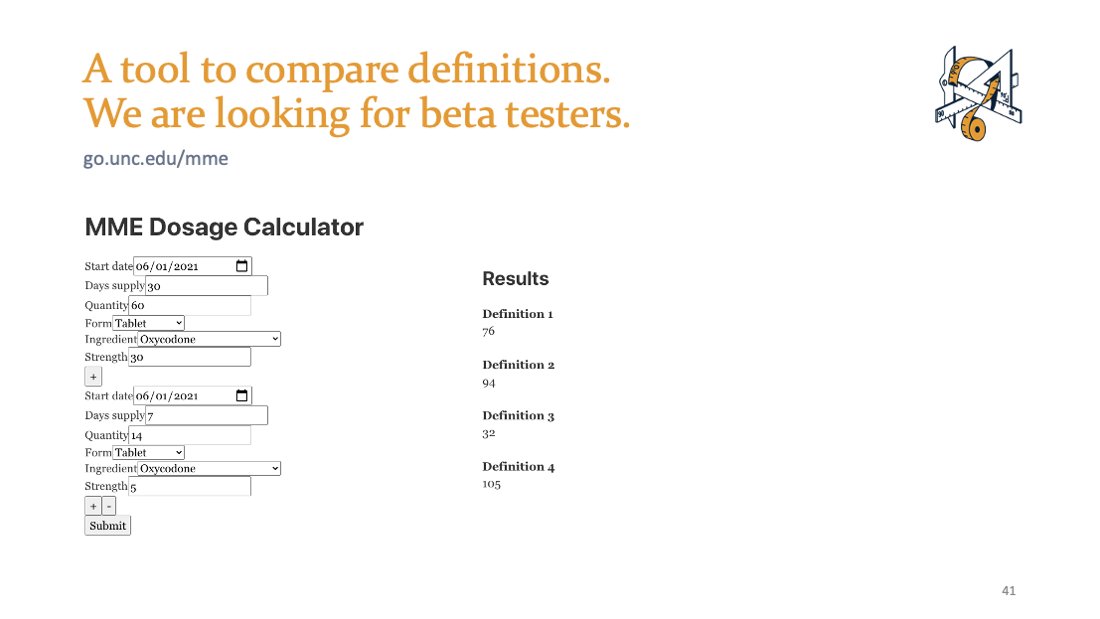
We are building a tool for research decisions. We need beta testers! DM or email me if you're interested. 
"And while everyone debates whether the MME limit was the right thing to do, we are forced to live by it, because medical personnel treat guidelines as mandates. So we wait. And we suffer. And we hope it will all get sorted so we can get the care we need.” Liz Joniak-Grant 

Or, as one patient put it to me when I showed them the results: "It's f--king arithmetic. Get it right."
Limitations of our study are here. We don't recommend using these conversion factors naively for clinical decisions, either. 

If a study doesn't sufficiently define how they calculated MME per day, I don't read the results.
These results will be published in @ClinJrnlPain (Clinical Journal of Pain) in a couple of weeks. Paper has been accepted and proofs are being typeset one last round. And yes, it'll be free open access. In the meantime, check out OpioidData.org for details.
Thanks to our talented illustrator-in-residence @brittainpeck for breathing visual life into technical medical concepts. Feel free to use any images or slides. 



In closing, if you are a researcher, please please please state your definition. Feel free to reuse our equations or slides. In this way, we can be allied with patients to reduce the most harm, with the best information.
Key message: Subtle choices have big consequences.
We offer proof that daily MME is not a standardized clinical metric.
Let us know what you think! Thanks for reading.
Follow co-authors
@chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey @Jungjun_Bae
We offer proof that daily MME is not a standardized clinical metric.
Let us know what you think! Thanks for reading.
Follow co-authors
@chris_delcher @yanningwang @AlanKinlaw @ToskaCooper @BrookeChidgey @Jungjun_Bae

• • •
Missing some Tweet in this thread? You can try to
force a refresh