
It's #ReadAlong Day!
@BrackenGrissom will #TakeOver our account at 12 pm ET.
Follow this thread for live tweets as we #ReadAlong: Latz & Case, 1992 (journals.uchicago.edu/doi/pdf/10.230…)
Drop your questions and comments in this thread.
Happy Reading! 🧵(1/n)
@BrackenGrissom will #TakeOver our account at 12 pm ET.
Follow this thread for live tweets as we #ReadAlong: Latz & Case, 1992 (journals.uchicago.edu/doi/pdf/10.230…)
Drop your questions and comments in this thread.
Happy Reading! 🧵(1/n)

@BrackenGrissom: Hi everyone! I am really excited to participate in my very first #ReadAlong with @BiolBulletin. My name is Heather Bracken-Grissom and I am an evolutionary marine biologist obsessed with decapod crustaceans. (2/n)
I started studying decapods as an undergraduate at UC-Santa Barbara and continued straight into my present position @FIU. (3/n)
I am the Assistant Director of Coastlines and Oceans in @FIUEnvironment and an Associate Professor in the Department of Biology. Most of my recent research is focused on the discovery of deep-sea biodiversity, bioluminescence and vision. (4/n)
Hence the reason for my 1992 Latz & Case pick for the induction of bioluminescence in deep-sea shrimp! Before I get into that paper, I do need to acknowledge my deep connection to @BiolBulletin. (5/n)
I was first introduced to the journal during a research experience for undergrad (REU) experience I did in the Fall of 2001 with Dr. Michael Greenberg @Whitney_Lab. He was the director of @Whitney_Lab at the time he was ALSO the editor-in-chief for @BiolBulletin. (6/n)
I vividly remember him showing me the journal, thumbing through articles with me and explaining the journal’s purpose. It is crazy to think that here I am, nearly 20 years later, on the editorial board! (7/n)
In fact, I owe a lot of my current interests in marine invertebrates to Dr. Greenberg. I spent many hours stumbling around the Florida beaches and waterways searching through sand documenting the meiofauna in the area. (8/n)
Yep folks, that was my REU for an entire summer… discovering the tiny inverts that live in between sand grains. I was introduced to an incredible new world of kinorhynchs, gastrotrichs, worms-galore, ostracods, among other beauties. I was in love. Thanks Dr. Greenberg! (9/n)
Ok, back to the reason for my current selection. Fast forward to 2003, I am just starting graduate school with Dr. Darryl Felder at the University of Louisiana at Lafayette and over the next glorious 5 years (I am not being facetious, I LOVED grad school). (10/n)
I decided to dive into shrimp systematics, taxonomy, and phylogenetics. I did some stuff on snapping shrimp, described some new species, built a caridean phylogeny, but when I finished I remember Felder telling me, “you need to study sergestid shrimp!” (11/n)
At the time, I was like… nahhhhh, but have now realized, sergestids are where it is at!!!!! Just look at them…
📸 @DanteFenolio Sergestid shrimps (only B, C, E, F).
🔗Varela et al. 2021 doi.org/10.1093/jcbiol… (12/n)
📸 @DanteFenolio Sergestid shrimps (only B, C, E, F).
🔗Varela et al. 2021 doi.org/10.1093/jcbiol… (12/n)

Not only are they some of the most abundant species in the deep-pelagic, they also participate in the largest migration on our planet (this is called diel vertical migration) and have a remarkable diversity of light organs capable of producing bioluminescence. (13/n)
These light organs can be internal (called organs of Pesta) or external. The external light organs also come in different flavors and can have lenses or non-lensed. (14/n)
Check them out here...
📸 @DanteFenolio. The diversity of light organ (i.e., organ of Pesta) morphologies in family Sergestidae, Sergestes sensu lato (s.l.).
🔗@LoriSchweikert @AlexLDavis_ @sonkelab (doi.org/10.1002/ece3.6…) (15/n)
📸 @DanteFenolio. The diversity of light organ (i.e., organ of Pesta) morphologies in family Sergestidae, Sergestes sensu lato (s.l.).
🔗@LoriSchweikert @AlexLDavis_ @sonkelab (doi.org/10.1002/ece3.6…) (15/n)

Now, my lab is currently all about these little glowing marvels. We are trying to better understand the diversification of these light organs and their function in the deep sea. Let dive into this paper and see what we can learn. (16/n)
So, we are going to skip the Abstract because it gives away ALL the good stuff and dive right into the Introduction... (17/n)
The title of the paper reveals the major objective which we will eventually get into, inducing bioluminescence (which I will here forth write as BL for ease of typing) in deep-sea shrimp. But before we do, I need to explain the reason for the induction. (18/n)
Many deep-sea animals, including the “star” of the show (sergestids) have a secret camouflage strategy called counterillumination and this paper starts out introducing this phenomenon. (19/n)
Counterillumination is when the animals produce BL that is directed downwards so that can hide their silhouettes from predators below...
📸 Ian Alexander (CC BY-SA 4.0, commons.wikimedia.org/w/index.php?cu…) (20/n)
📸 Ian Alexander (CC BY-SA 4.0, commons.wikimedia.org/w/index.php?cu…) (20/n)

They do this to match that of downwelling sunlight so they better blend in with the ambient light surrounding them. (21/n)
I want you to imagine you are a fish swimming below a shrimp and looking up for a tasty snack.
Now look at this picture and tell me, which one would be more likely get eaten the top or bottom?
📸 Ian Alexander (CC BY-SA 4.0, commons.wikimedia.org/w/index.php?cu…) (22/n)
Now look at this picture and tell me, which one would be more likely get eaten the top or bottom?
📸 Ian Alexander (CC BY-SA 4.0, commons.wikimedia.org/w/index.php?cu…) (22/n)

Lastly, you might be asking WHY animals in the deep-sea even need to counterilluminate in the first place if they are always in the dark? (23/n)
Well the answer's that they are not...
Most deep-sea animals participate in the largest migration on the planet known as Diel Vertical Migration or DVM and this is when they vertically migrate, sometimes 100s to even 1000m, at night to feeding shallow waters. (24/n)
Most deep-sea animals participate in the largest migration on the planet known as Diel Vertical Migration or DVM and this is when they vertically migrate, sometimes 100s to even 1000m, at night to feeding shallow waters. (24/n)
During this migration they are exposed to downwelling light (sunlight, moonlight) and need to counterilluminate to protect themselves! (25/n)
SHRIMPGLO’ is the name of the game of survival!
So now that we understand the importance of counterillumination and why it's critical for animals in the deep-sea, including sergestid shrimp, lets move on... (26/n)
So now that we understand the importance of counterillumination and why it's critical for animals in the deep-sea, including sergestid shrimp, lets move on... (26/n)
Fun fact is that counterillumination has only been experimental demonstrated in a few animals, and Sergestes similis is one of them. (27/n)
We also know from that paper that BL is NOT produced in the dark and from a series of other papers that sergestids can control several aspects of the SHRIMPGLO. (29/n)
This includes things like length, intensity of emittance, angular distribution and a whole series of amazing papers have explored this. (30/n)
But the outstanding question is: “How is it controlled?"
And this gets us to the central objective of this paper. (31/n)
And this gets us to the central objective of this paper. (31/n)
What we do know:
1) Some type of visual input is needed to induce BL from the light organs.
2) BL comes from those structures I showed you earlier called Organs of Pesta. These are modifications of the hepatopancreas. (32/n)
1) Some type of visual input is needed to induce BL from the light organs.
2) BL comes from those structures I showed you earlier called Organs of Pesta. These are modifications of the hepatopancreas. (32/n)
3) Other deep-sea animals like squids and myctophid fish seems to be under neural control. (33/n)
What we still do not know:
1) How the organs of Pesta are innervated with the rest of the body.
2) At the time of this paper, if BL could actually be induced by chemical and electrical stimulation. (34/n)
1) How the organs of Pesta are innervated with the rest of the body.
2) At the time of this paper, if BL could actually be induced by chemical and electrical stimulation. (34/n)
So this is what the authors Latz and Case set off to do. Let's now move into the methods and materials to see how they did it! (35/n)
Just to restate the objective: The authors wanted to induce luminescence and study what can trigger the response AND how quickly the response is triggered to learn more about the control on counterillumination. (36/n)
Ok, so how the heck do you collect deep-sea shrimps!?
In this paper they used something called a midwater trawl. (37/n)
In this paper they used something called a midwater trawl. (37/n)
Here is a picture of my research team using Tammy Frank’s “mother tucker” to collect a bunch of midwater shrimp. (38/n) 

The trawls we use can be opened and closed at a certain depth so that you know exactly the depth the shrimp came from. (39/n)
It is also VERY important they be collected in the “dark” in light tight containers and in temperature controlled waters so that they are not exposed to damaging levels of light and sea temperatures. (40/n)
In this study (Latz & Case), shrimp were collected at night, on moonless nights (guessing cloud coverage?) and sorted under red light so that were not exposed to light. (41/n)
Fun Fact #2: most deep-sea animals cannot see red light so you can sort them under red light and it does not damage their visual structures. (41/n)
Fun Fact #3: sergestids are very fragile and if you look at them the wrong way after collection (j/k, but no really) they croak. So collection of live animals, like these, and keeping them alive is no trivial feat. (42/n)
I am not going to get into the specifics of the set up but only animals that were active were used and they first used downward light stimulus to invoke a response. (43/n)
I am guessing the amount of light to use was definitely a challenge. It is really cool to see previous studies had measured the light in the Santa Barbara Basin where these shrimp were collected and they emulated those. (44/n)
Side tangent: Latz and Case were in the Marine Science Institute @ucsantabarbara, where I ALSO received a degree in Aquatic Science. Sooo, gooo Gauchos! (45/n)
Back to the paper...
BL was measured from below. In order to view the output they needed to set up a mirror beneath the shrimp at a 45 degree angle. The setup sounds a little confusing so lets just take a look at Fig. 1.
📸 Fig 1. (46/n)
BL was measured from below. In order to view the output they needed to set up a mirror beneath the shrimp at a 45 degree angle. The setup sounds a little confusing so lets just take a look at Fig. 1.
📸 Fig 1. (46/n)

A) Shows a dorsal view of the shrimp being held in the chamber. B) Shows the ventral view of an organ of Pesta that has been dissected out.
The dark regions are the parts that glow. (47/n)
The dark regions are the parts that glow. (47/n)

In brief, the shrimp was set in the chamber, allowed to acclimate for 20 min, exposed to different intensity of light (which were ecologically relevant) and measurements of BL was taken from the luminescent organs. (48/n)
That summarizes the photic stimulus, onto the chemical part. I want to also show the set up in case anyone was interested in the complexity of the apparatus! It is definitely a cool rig.
📸 Schematic of apparatus used to measure intensity of BL during counterillumination. (49/n)
📸 Schematic of apparatus used to measure intensity of BL during counterillumination. (49/n)
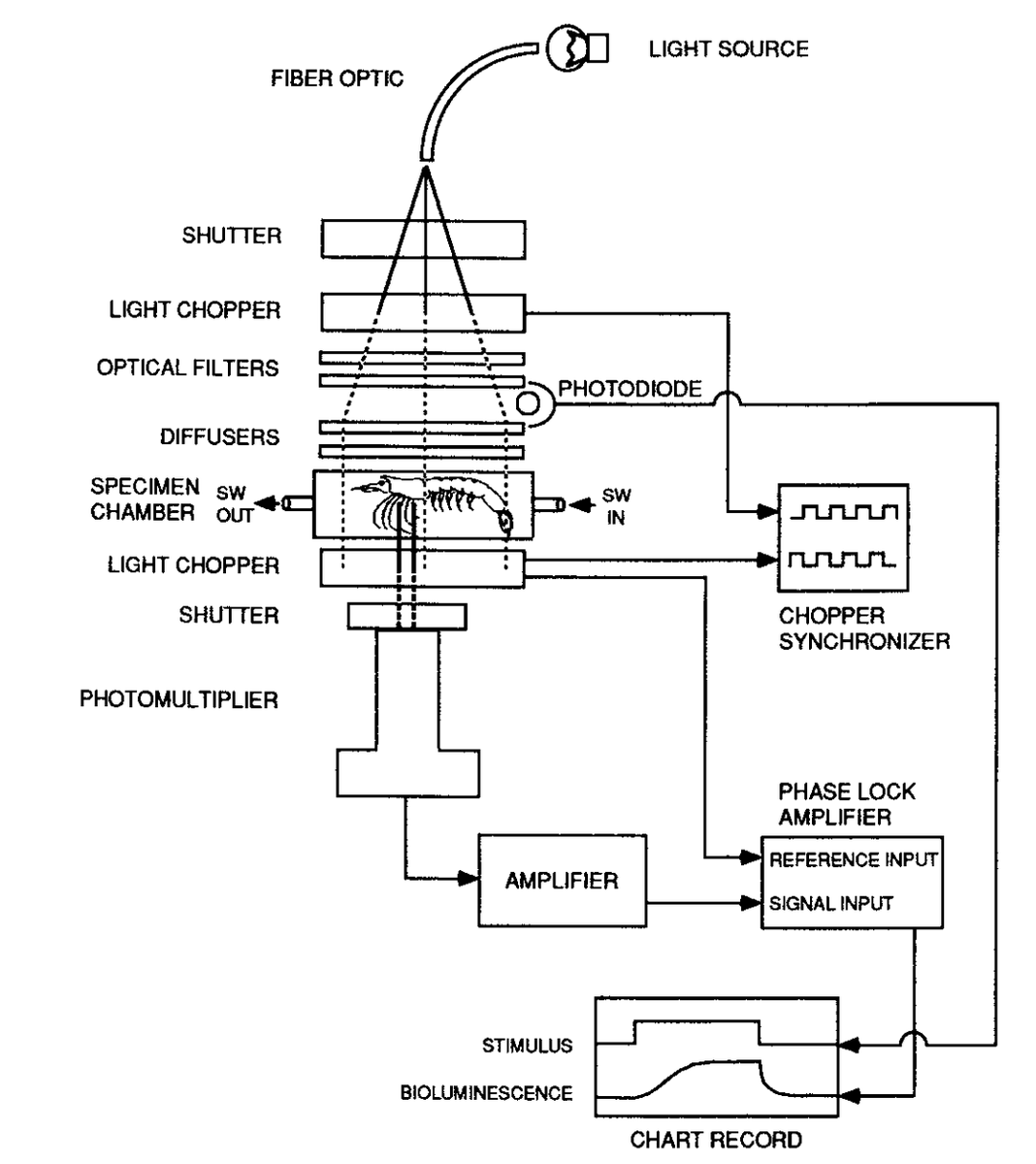
For the chemical test, the shrimp were collected and set up the same way. One big difference was the organs of Pesta were also dissected out and exposed to a series of chemical solutions. (50/n)
I am not going to list all the solutions that were prepared. But many included serotonin and serotonin-specific chemicals. Intact specimens and dissected organs of Pesta were submerged in the solutions and various responses were measured. (51/n)
Cool, so moving right along and lets get into what they found!!! (52/n)
First, lets touch on the most interesting things they found with the photic induction. It is important to note they did find a difference in the responses of previously untested animals to those that had already counterilluminated. (53/n)
Latz and Case describe a “typical counterillumination” response reaching half intensity around 13 seconds and a steady state at 25 seconds, whereas previously untested animals took much longer. (54/n)
In this study, those animals that were dark-adapted and untested previously, it took 13 minutes to reach half intensity and 25 minutes to reach max light output. (55/n)
This study also confirmed the light was actually emitted from the organs of Pesta, which may seem like a small feat, but really important to pinpoint the exact location! (56/n)
An interesting thing to note here is that once the initial induction was completed, all subsequent photic stimulations were quick! So, the induction took some time but once turned “on” the response was much faster. (57/n)
Lastly, before moving on, it was also interesting to find out that the slow induction process needs to be “reset” after some time in darkness (1 hour in this study). (58/n)
Lets just take a quick look at what this process may have looked like!
This is the ventral view so imagine you are below the animal looking up at it. You can clearly see those organs of Pesta getting brighter and brighter.
📸 Fig 4. (59/n)
This is the ventral view so imagine you are below the animal looking up at it. You can clearly see those organs of Pesta getting brighter and brighter.
📸 Fig 4. (59/n)

Do you also see that crazy posterior organs in the shape of a circle?!! I have some fun facts about those... (60/n) 

Fun Fact#4: sergestids have species-specific patterning of their organs of Pesta!
For example, the posterior organs can be uni-lobed, bi-lobed, tai-lobed or fringed. This is an example of a fringed pattern. (61/n)
For example, the posterior organs can be uni-lobed, bi-lobed, tai-lobed or fringed. This is an example of a fringed pattern. (61/n)
Remember that picture I showed you earlier? Here it is again to get a better look.
📸@DanteFenolio. @LoriSchweikert @AlexLDavis_ @sonkelab.
🔗 doi.org/10.1002/ece3.6… (62/n)
📸@DanteFenolio. @LoriSchweikert @AlexLDavis_ @sonkelab.
🔗 doi.org/10.1002/ece3.6… (62/n)

When I collect these beauties, I use their organ patterns, alongside rostral characters and tail fan characters to help identify them in the field! (63/n)
In this paper by @LoriSchweikert et al. we wanted to see if they shrimp could actually use the organ-specific patterns for con-specific recognition.
🔗 doi.org/10.1002/ece3.6… (64/n)
🔗 doi.org/10.1002/ece3.6… (64/n)
In other words “how well can they see?”
Turns out they can’t see very-well! Check it out here.
📸 Simulated visual perception of organs of Pesta based on the spatial resolution estimates across three species. @LoriSchweikert et al.
🔗 doi.org/10.1002/ece3.6… (65/n)
Turns out they can’t see very-well! Check it out here.
📸 Simulated visual perception of organs of Pesta based on the spatial resolution estimates across three species. @LoriSchweikert et al.
🔗 doi.org/10.1002/ece3.6… (65/n)

Although, we learned they do not have much resolution we did predict that they can detect bioluminescence over ecologically relevant distances (< 1 to ~6 m) and we have some fun work in the pipeline coming soon! Stay tuned. (66/n)
Now, back to the chemical induction and wrap up...
Result from chemical induction = Shrimp love serotonin too!
It made them glow, which made me smile 😊 (67/n)
Result from chemical induction = Shrimp love serotonin too!
It made them glow, which made me smile 😊 (67/n)
But seriously, all the solutions they made, the one that worked just as well as all the others was “serotonin alone.”
📸 Fig 5. The effect of chemical treatment on bioluminescence. (68/n)
📸 Fig 5. The effect of chemical treatment on bioluminescence. (68/n)

One interesting last results that was found was that “eyestalk ablation” (yes, I know sounds very scary) produced immediate BL. Here, in Fig. 7 this shows the effect of serotonin in intact and eyestalk less animals.
📸 Fig 7. Effect of bilateral eyestalk ablation on BL. (69/n)
📸 Fig 7. Effect of bilateral eyestalk ablation on BL. (69/n)

Ok, now to sum it all up! A few major take home points that I found really interesting:
1) The induction process took a relatively long time for sergestids that were in the dark and not previously tested. (70/n)
1) The induction process took a relatively long time for sergestids that were in the dark and not previously tested. (70/n)
And this was different from the previous counterillumination response noted by Warner et al. 1979 (doi.org/10.1126/scienc…).
It took 25 minutes to reach steady maximum level of SHRIMPGLO. (71/n)
It took 25 minutes to reach steady maximum level of SHRIMPGLO. (71/n)
2) Once induced, the counterillumination response was much faster and typical of what has previously been reported.
Latz and Case suggest these varied responses “could” indicate dual control mechanisms, one being neural and one being hormonal. (72/n)
Latz and Case suggest these varied responses “could” indicate dual control mechanisms, one being neural and one being hormonal. (72/n)
3) Evidence for neural induction was the fact that eyestalk squishing caused an immediate response and similar “quick” responses have been seen in deep-sea fishes. (73/n)
The authors state they see no evidence for organ innervation, BUT I am hoping our research team can solve that in the near future. We have several people working on this problem as we speak 😊 (74/n)
4) Evidence for hormonal control is supported by the long induction process similar to that of crustacean chromatophores (pigment cells). It was also induced by serotonin and evidence related to the eye experiments. (75/n)
Lastly, the reasons for the slow induction is not know, but several interesting speculations are made. (76/n)
They suggest that on moonless nights (remember these shrimp were collected under these conditions) the uninduced condition would be beneficial, because this would prevent BL responses from surrounding animals. (77/n)
It is interesting to note some similarity to other crustaceans, like euphausiids.
Finally, all data raises more questions than they answer, as do most experiments. (78/n)
Finally, all data raises more questions than they answer, as do most experiments. (78/n)
First, some control seems to be coming from the eyes. However, eyestalkless animals also responded, suggesting an additional control site. And finally, light emission from dissected out organs of Pesta suggested another possible control. (79/n)
Overall, and to wrap this ALL up, there seems to be close coupling of vision and BL, but many questions surrounding the control of BL are still outstanding and more work is to be done! (80/n)
I hope you all enjoyed this “tweetnado” and thank you for joining me and @BiolBulletin for this #ReadAlong. (fin)
Please unroll @threadreaderapp
• • •
Missing some Tweet in this thread? You can try to
force a refresh




