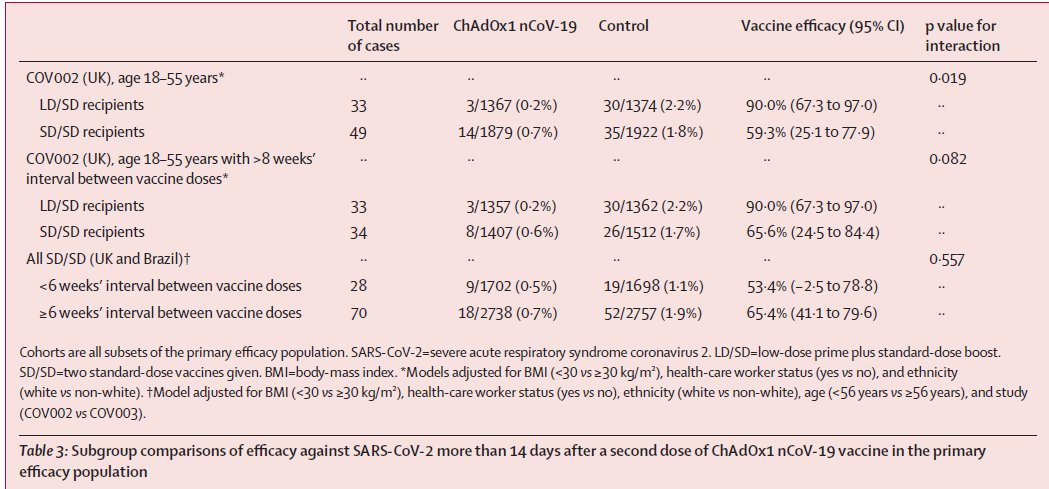
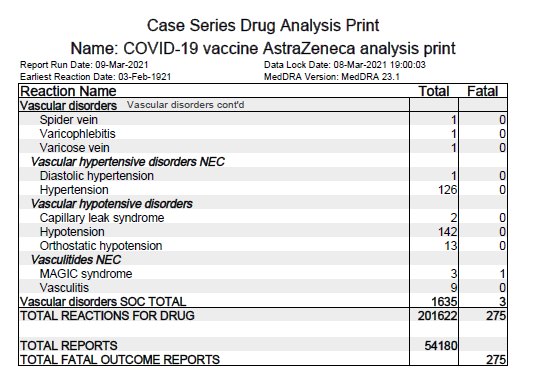
What do we know about ZyCoV-D, a plasmid DNA vaccine candidate against symptomatic COVID19 by Zydus Cadila? Thread.
1. Results of pre-clinical studies on animals before July 2020 put in public domain in 2021:
biorxiv.org/content/10.110… 26 Jan 2021
biorxiv.org/content/10.110… 3 Feb 2021

1. Results of pre-clinical studies on animals before July 2020 put in public domain in 2021:
biorxiv.org/content/10.110… 26 Jan 2021
biorxiv.org/content/10.110… 3 Feb 2021


2. Permission granted by DCGI on 2 Jun 2021 to initiate human trials adaptive Phases I & II.
Safety profile of 7 days after 1st dose of ZyCoV-D needed to be reviewed by DSMB & CDSCO before go ahead for Phase 2.
Some issues w/ these permissions are:
a) ZyCoV-D needs 3 doses for


Safety profile of 7 days after 1st dose of ZyCoV-D needed to be reviewed by DSMB & CDSCO before go ahead for Phase 2.
Some issues w/ these permissions are:
a) ZyCoV-D needs 3 doses for



completion (at day 0, day 28, day 56).
b) Vaccine candidate platform/tech is new for humans. 7 days for such a new vaccine candidate is too short period for monitoring. SAEs could occur based on dosages as well & some type of events may take longer to manifest.
c) Phase I trial
b) Vaccine candidate platform/tech is new for humans. 7 days for such a new vaccine candidate is too short period for monitoring. SAEs could occur based on dosages as well & some type of events may take longer to manifest.
c) Phase I trial

conducted at site owned by Zydus Cadila itself: Zydus Research Centre, Ahemdabad, Gujarat
DSMB is also constituted by pharma sponsor, so there's no "independence" either
Very objective of clinical trials are to reduce biases as much as possible but all concerns disregarded.
DSMB is also constituted by pharma sponsor, so there's no "independence" either
Very objective of clinical trials are to reduce biases as much as possible but all concerns disregarded.

Phase I trial started on July 15 & marketed as being over before 5 August, though participants had just received 1 dose of 3-dose regimen.
Phases 1 & 2 sample size of ~1000 (CTRI shows 1048 participants in total, ctri.nic.in/Clinicaltrials…).
Phase 2 began on 6 Aug 2020.
3 out of

Phases 1 & 2 sample size of ~1000 (CTRI shows 1048 participants in total, ctri.nic.in/Clinicaltrials…).
Phase 2 began on 6 Aug 2020.
3 out of


9 trial sites shown registered for Phase I/II are owned by Zydus Cadila.
3. On 2 Nov 2020, Zydus Cadila informed that for Phase-II clinical trials, they already completed enrolment & dosing of all 1000 subjects. Immunogenicity evaluation was going on then.
By Nov 2020 they also

3. On 2 Nov 2020, Zydus Cadila informed that for Phase-II clinical trials, they already completed enrolment & dosing of all 1000 subjects. Immunogenicity evaluation was going on then.
By Nov 2020 they also


had initiated development of recombinant measles vector construct expressing spike protein of 2019 nCov, which is a is a SARS-CoV-2 during the quarter, 2nd COVID19 vaccine candidate "ZyCoV-MV" of Zydus Cadila.
[Note: On 27 May 2021, Zydus informs that they've received approval
[Note: On 27 May 2021, Zydus informs that they've received approval

from the RCGM to carry out the preclinical and/or safety toxicity studies during the quarter]
4. On 24 Dec 2020, Zydus Cadila claimed that ZyCoV-D was found to be safe & immunogenic based on Phase 1/2 trial data submitted to DCGI.
Claimed Phase 3 trial permission on 3 Jan 2021.

4. On 24 Dec 2020, Zydus Cadila claimed that ZyCoV-D was found to be safe & immunogenic based on Phase 1/2 trial data submitted to DCGI.
Claimed Phase 3 trial permission on 3 Jan 2021.


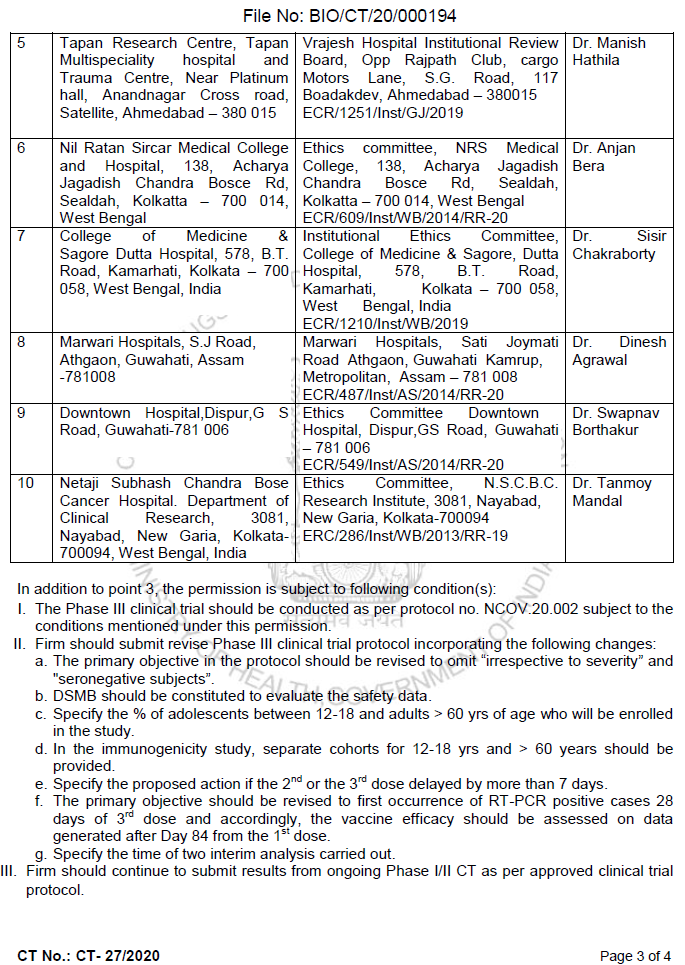
On 4 Jan 2021, DCGI issued permission to conduct clinical trial of ZyCoV-D ctri.nic.in/Clinicaltrials… [58 trial sites mentioned as of today in CTRI regd.]
Some trial sites in Phase 3 are owned by Zydus Cadila, while some would fall under "weird" category.




Some trial sites in Phase 3 are owned by Zydus Cadila, while some would fall under "weird" category.
https://twitter.com/das_seed/status/1362146365539831812



5. 39th Annual JP Morgan Health Care Conference, 11–14 Jan 2021, Zydus Cadila projected
a) expectation to deliver 100-150 million doses of ZyCoV-D by 2021 end🤦
b) Phase I/II Study on 1048 subjects
(Initial Phase I safety data for 7 days post 1st dose on how many subjects?🤔)
a) expectation to deliver 100-150 million doses of ZyCoV-D by 2021 end🤦
b) Phase I/II Study on 1048 subjects
(Initial Phase I safety data for 7 days post 1st dose on how many subjects?🤔)

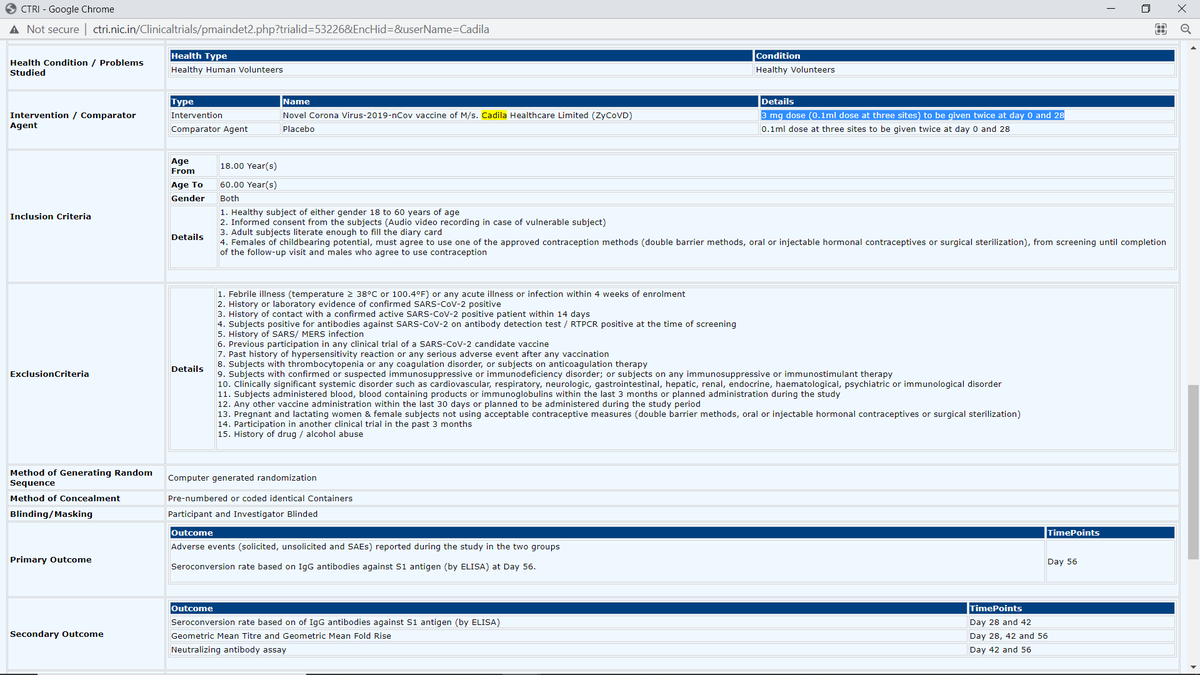
6. On 3 Feb 2021, SEC recommends permission to conduct Phase I/II clinical trial of ZyCoV-D (3 mg dose (0.1ml dose @ 3 sites) given twice at 28 days apart). cdsco.gov.in/opencms/opencm…
ctri.nic.in/Clinicaltrials…
CTRI description:
Sample size: 150
1st enrollment: 19/03/2021

ctri.nic.in/Clinicaltrials…
CTRI description:
Sample size: 150
1st enrollment: 19/03/2021

On 3 Feb 2021, SEC also recommended permission for extension of Phase I part of ongoing Phase I/II
clinical trial for assessment of persistence of NAbs. ctri.nic.in/Clinicaltrials…
CTRI details
Sample size: 20 (18 enrolled)
1st enrollment: 22/02/2021
Study completion: 04/03/2021
clinical trial for assessment of persistence of NAbs. ctri.nic.in/Clinicaltrials…
CTRI details
Sample size: 20 (18 enrolled)
1st enrollment: 22/02/2021
Study completion: 04/03/2021

7. On 16 Feb 2021, Gautam Budh Nagar health department conducted search at Gopal Pathology Lab (GPL, Dadri). It had received reports/complaints that GPL was conducting unauthorized vaccination camp.
Upon search they found that Flores Hospital, a ZyCoV-D trial site, had

Upon search they found that Flores Hospital, a ZyCoV-D trial site, had


actually given IP (investigational products, which could be ZyCoV-D or placebo probably since this Phase 3 trial is claimed to be double-blinded placebo controlled) vials to GPL.
GPL was duping public by claiming IP to be vaccine.
There's no news as to what actions were taken
GPL was duping public by claiming IP to be vaccine.
There's no news as to what actions were taken
against sponsor (Zydus Cadila), Flores Hospital, GPL.
What was Zydus Cadila doing?
What was Institutional Ethics Committee doing?
What was DSMB doing?
What did DCGI do about it?
How many other sites were involved in such malpractice to achieve target?
What was Zydus Cadila doing?
What was Institutional Ethics Committee doing?
What was DSMB doing?
What did DCGI do about it?
How many other sites were involved in such malpractice to achieve target?
https://twitter.com/das_seed/status/1349373244147642368
8. On 5 Feb 2021, Zydus Cadila
a) expected to jab ~15000 people of 1st dose of IP by mid-Feb & complete enrollment of participants in Phase 3 by end of Feb 2021
b) Main plant expected to be ready for commercial production of ZyCoV-D by Q1 of FY '22 (~12cr doses annual capacity)

a) expected to jab ~15000 people of 1st dose of IP by mid-Feb & complete enrollment of participants in Phase 3 by end of Feb 2021
b) Main plant expected to be ready for commercial production of ZyCoV-D by Q1 of FY '22 (~12cr doses annual capacity)


c) Current plants of production capacity 10 million to 20 million doses (annually). Ready for manufacturing from April 2021.
d) Looking for CMOs to add. produce 50-70 million doses.
[However, as briefed today, 1 Jul 2021, Zydus Cadila has not started stockpiling of ZyCoV-D yet]

d) Looking for CMOs to add. produce 50-70 million doses.
[However, as briefed today, 1 Jul 2021, Zydus Cadila has not started stockpiling of ZyCoV-D yet]


9. On 27 May 2021, Zydus Cadila on ZyCoVD-D:
a) put up a plant for production of large-scale mfg, expected ready for commercialization by end of June 2021
b) committed to initially start producing 1cr doses per month
c) vaccine uses an intradermal device & has also secured


a) put up a plant for production of large-scale mfg, expected ready for commercialization by end of June 2021
b) committed to initially start producing 1cr doses per month
c) vaccine uses an intradermal device & has also secured



whole supply chain for the device as well
d) completely built capabilities to product both drug substance & product & the devices. No concern anywhere between 1-2 crores doses per month
e) 1st interim analysis when 79 cases of COVID19 occur in Phase 3 for efficacy (target: 158)

d) completely built capabilities to product both drug substance & product & the devices. No concern anywhere between 1-2 crores doses per month
e) 1st interim analysis when 79 cases of COVID19 occur in Phase 3 for efficacy (target: 158)


f) submitted the data to publish the results of ZyCoV-D Phase-I clinical trials and the results to be published soon in a peer review journal
g) More than 1000 children enrolled in Phase 3 trial.
10. ~20 May 2021, @DeccanHerald reported 2nd dose to kids b/w age 12 to 18 years

g) More than 1000 children enrolled in Phase 3 trial.
10. ~20 May 2021, @DeccanHerald reported 2nd dose to kids b/w age 12 to 18 years


was underway at Jeevan Rekha Hospital in Belagavi, only trial site for kids in Karnataka.
11. Govt submits affidavit in Supreme Court on 26 June projecting 5 crore doses of ZyCoV-D would be available (efficacy trial on 3 doses regimen) b/w Aug-Dec 2021.
At this point, Zydus

11. Govt submits affidavit in Supreme Court on 26 June projecting 5 crore doses of ZyCoV-D would be available (efficacy trial on 3 doses regimen) b/w Aug-Dec 2021.
At this point, Zydus


Cadila also claimed to have done abridged trial of sample size 150 for 2 doses regimen (each dose of 3mg) of ZyCoV-D.
Govt in its affidavit also mentions ZyCoV-D to be available for kids (12+ years) in near future after emergency use authorization.
12. On 1 July 2021, Zydus
Govt in its affidavit also mentions ZyCoV-D to be available for kids (12+ years) in near future after emergency use authorization.
12. On 1 July 2021, Zydus

Cadila submits "khhichdi" application to CDSCO for emergency approval of ZyCoV-D:
Results/data of both types of dosing regimens (3 doses & 2 doses)- analyzed adults data from all phases, incl. immunogenicity data of few kids (w/ 3 doses regimen) from Phase 2. Opacity remains.



Results/data of both types of dosing regimens (3 doses & 2 doses)- analyzed adults data from all phases, incl. immunogenicity data of few kids (w/ 3 doses regimen) from Phase 2. Opacity remains.




Dr Sharvil Patel, Zydus Cadila on 1 July 2021:
Phase I is almost there in pre-print. We are now working on Phase 2 data... Phase 3 in print will take time, need to complete. Can't go w/ partial data, will take @ least 4-6months.
[Phase 3 trial approved w/o Phase 1/2 analysis🙈]
Phase I is almost there in pre-print. We are now working on Phase 2 data... Phase 3 in print will take time, need to complete. Can't go w/ partial data, will take @ least 4-6months.
[Phase 3 trial approved w/o Phase 1/2 analysis🙈]
Immunogenicity analysis on 12-18 years kids from Phase 3 not yet started. Will take some weeks.
Onus on CDSCO to scrutinize & compute analyses of Phase 2 data & interim Phase 3 data on adults. Zydus Cadila believes large amount of data submitted to seek approval for age 12+ yrs.
Onus on CDSCO to scrutinize & compute analyses of Phase 2 data & interim Phase 3 data on adults. Zydus Cadila believes large amount of data submitted to seek approval for age 12+ yrs.
Zydus Cadila, 1 Jul 2021:
No stockpiling of ZyCoV-D yet.
In smaller plants, will try to produce ~4-5 lakh doses per month. Main plant just coming up now for large commercial production (~1cr doses per month) from mid-August if all goes well.
[Final analysis to be on 158 cases]
No stockpiling of ZyCoV-D yet.
In smaller plants, will try to produce ~4-5 lakh doses per month. Main plant just coming up now for large commercial production (~1cr doses per month) from mid-August if all goes well.
[Final analysis to be on 158 cases]
• • •
Missing some Tweet in this thread? You can try to
force a refresh