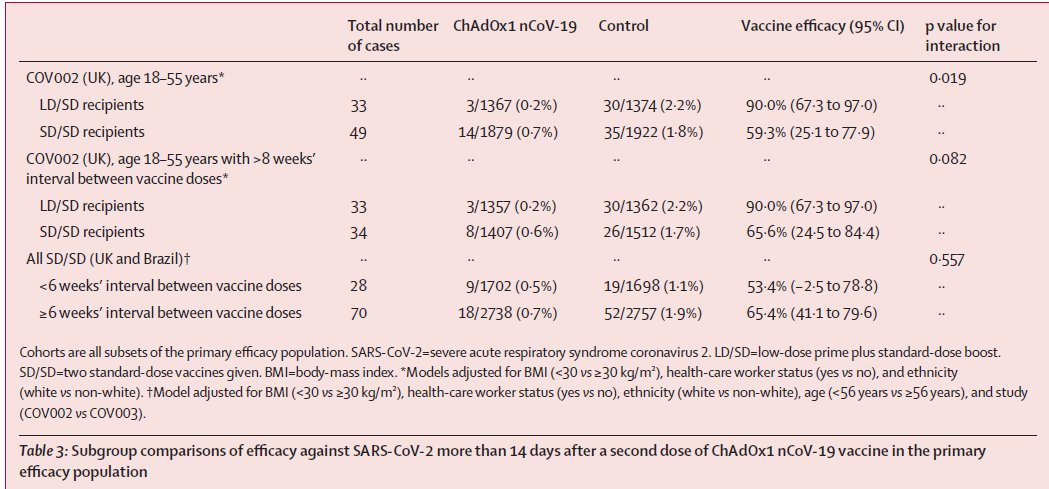
Should Indians be concerned about commercial COVAXIN deals by @BharatBiotech?
@ICMRDELHI & @BharatBiotech have MoU on COVAXIN and also share Intellectual Property rights.
ICMR gets 5% on net sales of COVAXIN as royalty from BBIL.
Partial COVAXIN trials & mfg support from

@ICMRDELHI & @BharatBiotech have MoU on COVAXIN and also share Intellectual Property rights.
ICMR gets 5% on net sales of COVAXIN as royalty from BBIL.
Partial COVAXIN trials & mfg support from
https://twitter.com/ShobhanSaxena/status/1410741584979644419


Indian govt.
Not to forget super-extraordinary regulatory authorization to COVAXIN w/o Phase 3 trial data that allowed BBIL to opened door for early sales of COVAXIN in India & some other nations.
During early days, COVAXIN consignments only had BBIL's logo but not of ICMR.
Not to forget super-extraordinary regulatory authorization to COVAXIN w/o Phase 3 trial data that allowed BBIL to opened door for early sales of COVAXIN in India & some other nations.
During early days, COVAXIN consignments only had BBIL's logo but not of ICMR.
However, there're COVAXIN consignments featuring logos of both developers, ICMR & BBIL, from March.
Commercial supply of 2 lakh COVAXIN doses to Mauritius on 19 March had logos of ICMR & BBIL w/ sign of #VaccineMaitri by Indian govt.
Was ICMR logo because of int'l shipment? No.

Commercial supply of 2 lakh COVAXIN doses to Mauritius on 19 March had logos of ICMR & BBIL w/ sign of #VaccineMaitri by Indian govt.
Was ICMR logo because of int'l shipment? No.


There're COVAXIN consignments supplied w/n India during March w/ logos of both developers, BBIL & ICMR.
No rationale to why logo of ICMR initially not included. As ICMR is co-developer, its logo should continue to feature in COVAXIN consignments, right?


No rationale to why logo of ICMR initially not included. As ICMR is co-developer, its logo should continue to feature in COVAXIN consignments, right?
https://twitter.com/malini_aisola/status/1410865211200851969

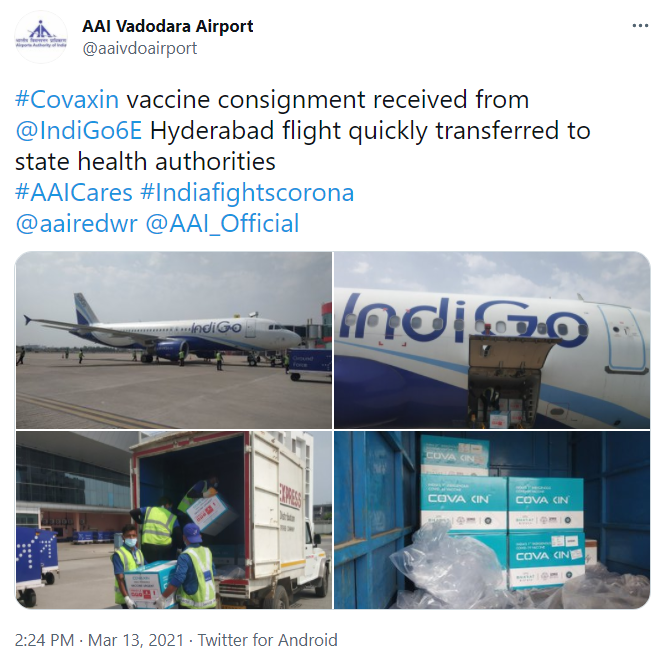
Well, not sure what unfolded but there are consignments being supplied in May which don't have logo of ICMR.
There's also augmentation of mfg capacity for COVAXIN production under Mission COVID Suraksha by Indian govt. 3 PSUs identified to support BBIL.



There's also augmentation of mfg capacity for COVAXIN production under Mission COVID Suraksha by Indian govt. 3 PSUs identified to support BBIL.
https://twitter.com/das_seed/status/1397822032163885056



Even boxes of vials don't contain logo of ICMR (left pic tweeted by @SuchitraElla, 21 June '21)
COVAXIN development & mfg partially supported by Govt.
Commercial deals also facilitated by Govt.
COVID19 vaccines are public good.
Bizarre for Govt & media to be mute spectator.

COVAXIN development & mfg partially supported by Govt.
Commercial deals also facilitated by Govt.
COVID19 vaccines are public good.
Bizarre for Govt & media to be mute spectator.


🤦♀️On 21 May 2021, @SuchitraElla said
a) @BharatBiotech reached out to ICMR-NIV only for SARS-CoV-2 strain. There was no technology transfer.
b) From there, it was developed in-house, completely funded in-house & taken through all testing, animal, toxicology & human testing &
a) @BharatBiotech reached out to ICMR-NIV only for SARS-CoV-2 strain. There was no technology transfer.
b) From there, it was developed in-house, completely funded in-house & taken through all testing, animal, toxicology & human testing &
everything was done as per int'l norm & protocols of drug development/discovery.
c) ICMR-NIV, ICMR & BBIL have collaborated on trials in large animals (monkeys, hamsters).
🤨On 2 July 2021, Phase 3 trial paper mentions only BBIL as developer of COVAXIN.


c) ICMR-NIV, ICMR & BBIL have collaborated on trials in large animals (monkeys, hamsters).
🤨On 2 July 2021, Phase 3 trial paper mentions only BBIL as developer of COVAXIN.
https://twitter.com/das_seed/status/1411069675623043072


• • •
Missing some Tweet in this thread? You can try to
force a refresh