Phase 3 trial results for COVAXIN (a double-blind, randomised, placebo-controlled trial). Thread.
medrxiv.org/content/10.110… 2 July 2021.
1. Vaccine efficacy against
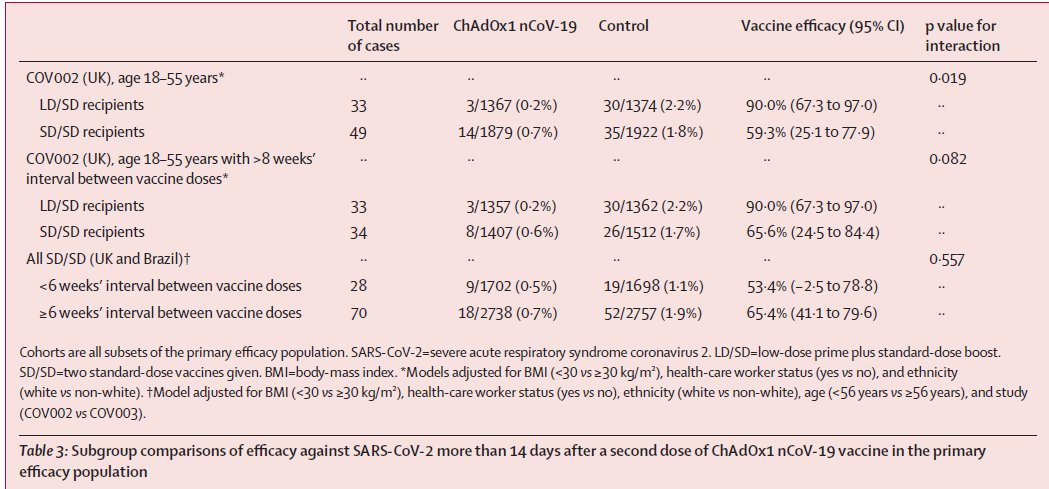
● Symptomatic COVID19+ve: 77.8% (95% CI: 65.2–86.4) w/ 130 cases (24 COVAXIN, 106 placebo)
medrxiv.org/content/10.110… 2 July 2021.
1. Vaccine efficacy against
● Symptomatic COVID19+ve: 77.8% (95% CI: 65.2–86.4) w/ 130 cases (24 COVAXIN, 106 placebo)

● Severe symptomatic COVID-19+ve: 93·4% (57·1–99·8) w/ 16 cases (1 COVAXIN, 15 placebo)
● Symptomatic COVID19+ve in age 18–59 yrs: 79·4% (66–88·2) w/ 109 cases (19 COVAXIN, 90 placebo)
● Asymptomatic COVID19+ve: 63·6% (29🤔–82·4) w/ 47(?) cases (13 COVAXIN+33 placebo=46🤦)

● Symptomatic COVID19+ve in age 18–59 yrs: 79·4% (66–88·2) w/ 109 cases (19 COVAXIN, 90 placebo)
● Asymptomatic COVID19+ve: 63·6% (29🤔–82·4) w/ 47(?) cases (13 COVAXIN+33 placebo=46🤦)


2. Vaccine efficacy against symptomatic COVID19+ve
● B.1.617.2 (Delta): 65·2% (33·1–83·0) w/ 50 cases (13 COVAXIN, 37 placebo)
● B.1.617.1 (Kappa): 90·1% (30·4–99·8) w/ 11 cases (1 COVAXIN, 10 placebo)
3. Total 79 variants (VOIs or VOCs) detected in 130 cases analyzed for
● B.1.617.2 (Delta): 65·2% (33·1–83·0) w/ 50 cases (13 COVAXIN, 37 placebo)
● B.1.617.1 (Kappa): 90·1% (30·4–99·8) w/ 11 cases (1 COVAXIN, 10 placebo)
3. Total 79 variants (VOIs or VOCs) detected in 130 cases analyzed for

vaccine efficacy. Among these 79 cases (18 COVAXIN, 61 placebo), 4 cases of severe COVID19+ve, all 4 of them in placebo.
4. Population enrolled in trial
● Placebo: 4254 ♀️, 8620 ♂️
COVAXIN: 4214 ♀️, 8665 ♂️
● At baseline (day 0): 213 COVID+ve, 7818 anti-SARS-CoV-2 IgG+ve.

4. Population enrolled in trial
● Placebo: 4254 ♀️, 8620 ♂️
COVAXIN: 4214 ♀️, 8665 ♂️
● At baseline (day 0): 213 COVID+ve, 7818 anti-SARS-CoV-2 IgG+ve.


● 15 deaths (5 COVAXIN, 10 placebo) in trial, none deemed related to COVAXIN or placebo:
5 deaths in COVAXIN: 1 cerebellar haemorrhage, 1 haemorrhagic stroke, 1 ovarian cancer w/ metasases, 1 sudden cardiac, 1 COVID19
10 deaths in placebo: 1 alcohol overdose, 1 myocardial

5 deaths in COVAXIN: 1 cerebellar haemorrhage, 1 haemorrhagic stroke, 1 ovarian cancer w/ metasases, 1 sudden cardiac, 1 COVID19
10 deaths in placebo: 1 alcohol overdose, 1 myocardial
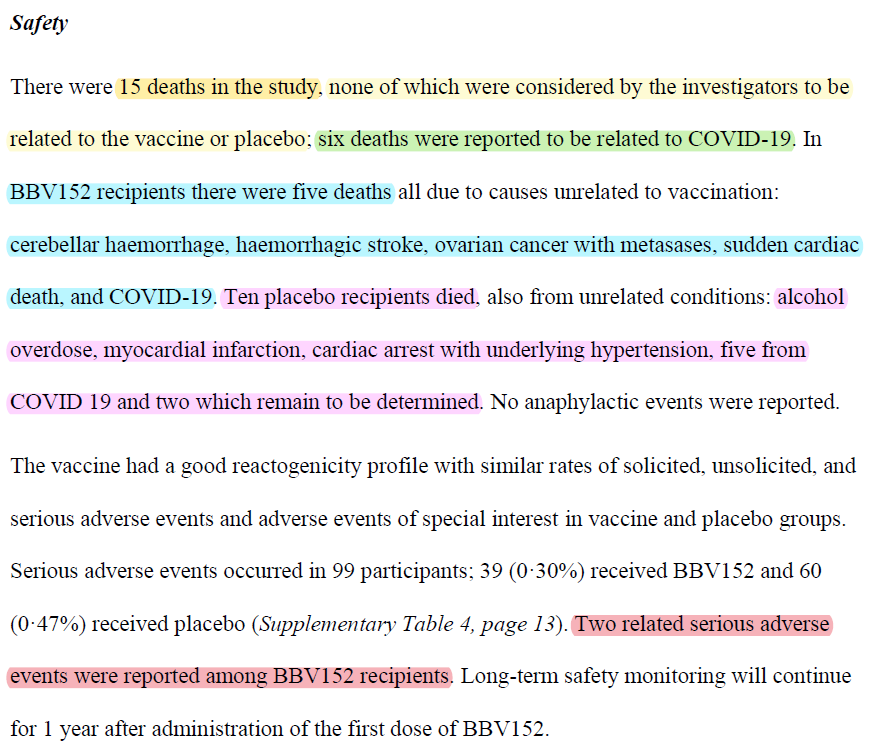

infarction, 1 cardiac arrest w/ hunderlying hypertension, 5 COVID19, 2 yet to be determined.
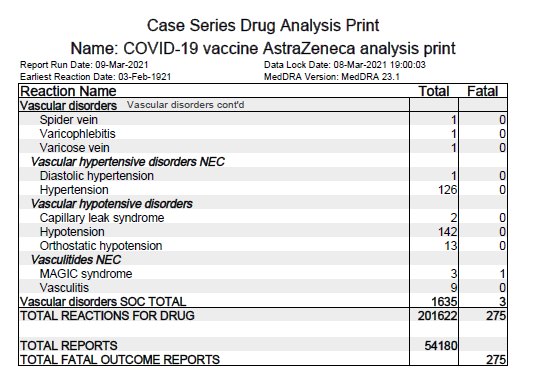
● Summary of AEs (Left pic)
● Incidences of solicited AEs after each dose (Right pic)
🤔In Safety, mentions
"2 related serious adverse events were reported among COVAXIN recipients"

● Summary of AEs (Left pic)
● Incidences of solicited AEs after each dose (Right pic)
🤔In Safety, mentions
"2 related serious adverse events were reported among COVAXIN recipients"


but later in Discussion mentions
"one possibly related serious adverse event in COVAXIN group was a case of immune thrombocytic purpura that occurred 39 days after the 2nd dose in a participant who was SARS-CoV-2 seropositive at baseline, which resolved in four days."
🤦♀️🤦.

"one possibly related serious adverse event in COVAXIN group was a case of immune thrombocytic purpura that occurred 39 days after the 2nd dose in a participant who was SARS-CoV-2 seropositive at baseline, which resolved in four days."
🤦♀️🤦.


Total people (🧑🤝🧑): 12879🧑🤝🧑 (12879 COVAXIN, 12874 placebo)
All AEs: 3194🧑🤝🧑 (1597 COVAXIN, 1597 placebo)🤨
Unsolicited AEs: 1098🧑🤝🧑 (489 COVAXIN, 609 placebo)🤨🤨
SAEs: 99🧑🤝🧑 (39 COVAXIN, 60 placebo)🤨🤨
Any immediate AE (w/in 30 mins of jab): 35🧑🤝🧑 (12 COVAXIN, 23 placebo)🤨🤨
All AEs: 3194🧑🤝🧑 (1597 COVAXIN, 1597 placebo)🤨
Unsolicited AEs: 1098🧑🤝🧑 (489 COVAXIN, 609 placebo)🤨🤨
SAEs: 99🧑🤝🧑 (39 COVAXIN, 60 placebo)🤨🤨
Any immediate AE (w/in 30 mins of jab): 35🧑🤝🧑 (12 COVAXIN, 23 placebo)🤨🤨

All Medically Attended AEs: 620🧑🤝🧑 (301 COVAXIN, 319 placebo)🤨🤨
AESIs: 46🧑🤝🧑 (23 COVAXIN, 23 placebo) 🤨🤨
[Looks like COVAXIN is safer than placebo🐒. Someone should calculate p-values.]
5. Puzzles on last jab timeline.
●On 7 Jan 2021, @BharatBiotech announced completion of

AESIs: 46🧑🤝🧑 (23 COVAXIN, 23 placebo) 🤨🤨
[Looks like COVAXIN is safer than placebo🐒. Someone should calculate p-values.]
5. Puzzles on last jab timeline.
●On 7 Jan 2021, @BharatBiotech announced completion of


enrollment for Phase 3.
Paper on Phase 3 trial states that b/w 16 Nov 2020 & 7 Jan 2021, 25798 participants recruited & randomized.
First dose of IP was given to participant on day of randomization itself (day 0). COVAXIN needs 2 doses to be given, dosing interval of 28 days.
Paper on Phase 3 trial states that b/w 16 Nov 2020 & 7 Jan 2021, 25798 participants recruited & randomized.
First dose of IP was given to participant on day of randomization itself (day 0). COVAXIN needs 2 doses to be given, dosing interval of 28 days.
Therefore, if the last person was enrolled & randomized on 7 Jan 2021 then person would have got 1st dose on 7 Jan & next dose in 1st week of Feb itself.
However, on 9 June 2021, @RachesElla claimed that the last participant received the 2nd dose around mid-March. Why & how?🤔
However, on 9 June 2021, @RachesElla claimed that the last participant received the 2nd dose around mid-March. Why & how?🤔

6. Puzzles on conductance of trial in compliance w/ all ICH GCP guidelines.
For instances:
● Paper states regd at clinicaltrials.gov/ct2/show/NCT04… and "Volunteers were screened for eligibility based on their health status, includ. medical history, vital signs, &

For instances:
● Paper states regd at clinicaltrials.gov/ct2/show/NCT04… and "Volunteers were screened for eligibility based on their health status, includ. medical history, vital signs, &
https://twitter.com/das_seed/status/1349373244147642368

physical examination results. Eligible participants provided signed & dated informed consent forms at enrolment"
In late Dec 2020 & early Jan 2021, huge misconduct in trial at Bhopal's site, People’s Hospital, came to light, see fmesinstitute.org/wp-content/upl…
In late Dec 2020 & early Jan 2021, huge misconduct in trial at Bhopal's site, People’s Hospital, came to light, see fmesinstitute.org/wp-content/upl…
https://twitter.com/das_seed/status/1349373284329140226
Many illiterate people who were unaware of trials were recruited in name of "vaccine" against COVID19. As per protocol, these people shouldn't have been recruited as they couldn't give signed & dated consent form (we can forget about informed consent). Even medical conditions
were not considered.
An individual w/ ovarian cancer got enrolled in trial, who unfortunately died.
People's Univ, Bhopal is one of 14 sites out of 25 trial sites in Phase 3 whose PI is coauthor w/ team from BBIL, ICMR, ICMR-NIV, & a stat consultancy.😱


An individual w/ ovarian cancer got enrolled in trial, who unfortunately died.
People's Univ, Bhopal is one of 14 sites out of 25 trial sites in Phase 3 whose PI is coauthor w/ team from BBIL, ICMR, ICMR-NIV, & a stat consultancy.😱
https://twitter.com/das_seed/status/1349385209632927745


● Unsettling efficacy/safety analysis
a) 2nd interim analysis cut-off was 87 cases but due to COVID19 surge 127 cases were accrued & analyzed by 21 April 2021, efficacy estimate 78% (CI: 61–88) a/c to @BharatBiotech & @ICMRDELHI.
Target of 130 cases for final analysis. Twist

a) 2nd interim analysis cut-off was 87 cases but due to COVID19 surge 127 cases were accrued & analyzed by 21 April 2021, efficacy estimate 78% (CI: 61–88) a/c to @BharatBiotech & @ICMRDELHI.
Target of 130 cases for final analysis. Twist
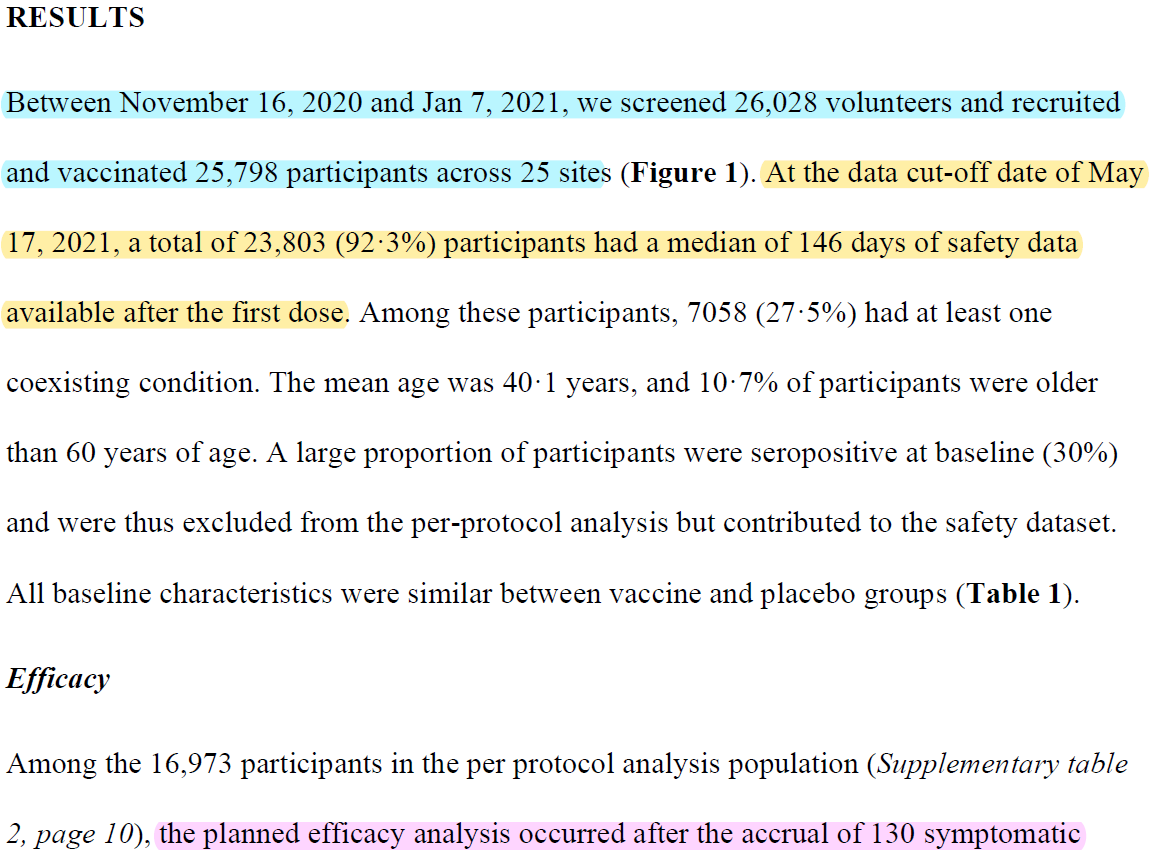

is that cut-off date for safety analysis is 17 May 2021.
Cases surging more rapidly during late April & early May (enormously), more cases must have been accrued in Phase 3 trial participants during this period than mere 3 added to already analyzed 127 cases.
What was actual
Cases surging more rapidly during late April & early May (enormously), more cases must have been accrued in Phase 3 trial participants during this period than mere 3 added to already analyzed 127 cases.
What was actual

count of COVID19 disease among participants till 17 May is unknown. Paper gives no description of what actually "lost follow-ups" mean when contact/residence addresses are recorded during enrollment.
b) There's no info of how many deaths took place post 2-doses of IP. Did

b) There's no info of how many deaths took place post 2-doses of IP. Did


efficacy analysis against severe COVID19+ve constituted deaths, required ICU, or standard of care hospitalization (w/ or w/o oxygen supply) in COVAXIN/placebo aren't clarified.
Though efficacy analysis against COVID19 mortality is secondary endpoint. This leaves a critical
Though efficacy analysis against COVID19 mortality is secondary endpoint. This leaves a critical

question unanswered: were there no deaths after 2 weeks of 2nd dose of IP (COVAXIN or placebo)?
c) There's no explanation or discussion on as to why Unsolicited AEs, SAEs*, immediate AEs*, MMAEs were (*considerably) higher in placebo group than COVAXIN. What makes COVAXIN
c) There's no explanation or discussion on as to why Unsolicited AEs, SAEs*, immediate AEs*, MMAEs were (*considerably) higher in placebo group than COVAXIN. What makes COVAXIN

have a better safety profile than placebo (Phosphate buffered saline w/ Algel). There's no mentioning of what adverse events of concern were seen. When there was no anaphylaxis recorded, what kind of immediate AEs were seen, assuming that healthy participants w/ consent enroll. 

• • •
Missing some Tweet in this thread? You can try to
force a refresh