How do the various mutations within the SARS-CoV-2 variants impact vaccine-induced immunity? The amazing @carolilucas @VogelsChantal @InciYildirim11 led this study with with help from others to tackle this question - using 18 CoV-2 variants. (1/n)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
The study was designed to measure antibody and T cell immunity from people who were previously infected or not infected with SARS-CoV-2, before and after the 1st and 2nd doses of mRNA vaccines. Amazing effort by @InciYildirim11 @SaadOmer3 (2/n) 

Antibodies to the ancestral S1 and RBD were induced in both prev. infected and uninfected vaccinees. S/S1/RBD-specific IgG levels in response to vaccination were significantly higher in the previously infected compared to uninfected, as reported by others. (3/n) 



Next, neutralization activity of antibodies induced by the mRNA vaccines was measured through 50% plaque-reduction neutralization (PRNT50). Despite faster and higher NAb responses in previously infected, similar NAb levels were achieved after 2nd dose in both groups. (4/n) 

Notice that even though every vaccinated person generated anti-Spike IgG responses, 2 individuals failed to induce neutralizing Abs even after the 2 shots. We don’t know the underlying cause yet. (5/n)
Next we examined CD4 and CD8 T cell responses to spike and nucleocapsid proteins. mRNA vaccines induced robust activation of CD4 T cells in both previously infected and uninfected vaccinees against ancestral and P.1 spike. (6/n) 

In contrast, we found reduced CD8 T cell responses to the P.1 variant spike antigen compared to ancestral spike. This may be related to the various mutations in P.1 spike protein. Mutations are discussed below. (7/n) 
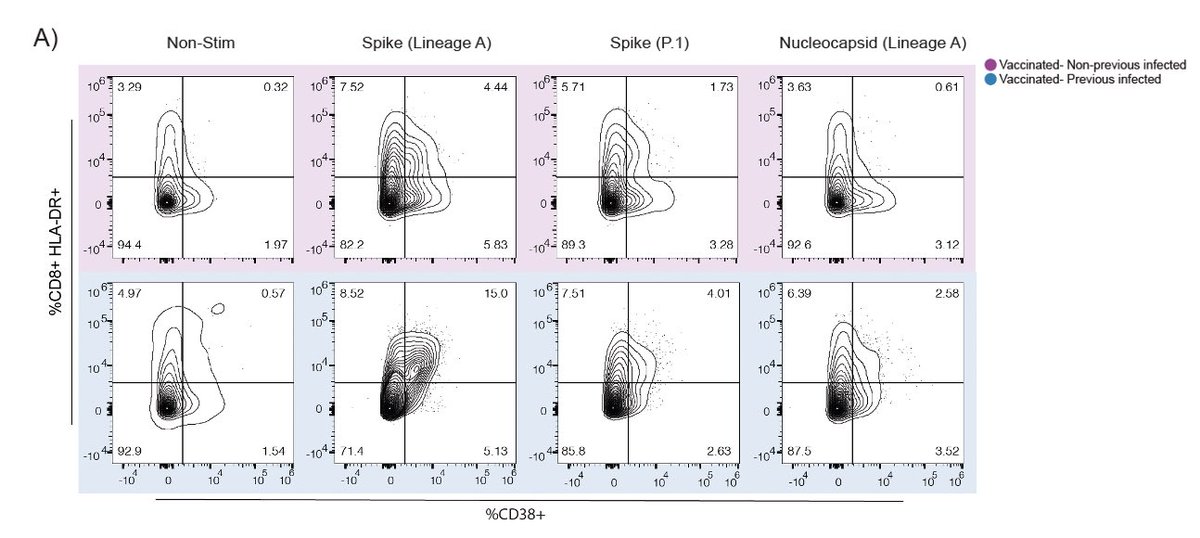
Note that other studies found no reduction in T cell responses to ancestral vs. VOC spike antigens in vaccinated people. Difference may be due to assays or hosts. (8/n)
Ad26 vaccine: nature.com/articles/s4158…
mRNA vaccines: sciencedirect.com/science/articl…
Ad26 vaccine: nature.com/articles/s4158…
mRNA vaccines: sciencedirect.com/science/articl…
How well do mRNA vaccine-induced antibodies work against variants? This is where having collaborators like @NathanGrubaugh is so powerful & enabling 💪🏼 He and his team have been surveying and studying every CoV2 variant in the community. (9/n)
https://twitter.com/nathangrubaugh/status/1415763895373996034
We’re so lucky to tag team with @VogelsChantal in the Grubaugh lab who identified & provided 16/18 variants studied here. @carolilucas painstakingly propagated these variants and @VogelsChantal sequenced and validated each isolate. Spectacular team work! (10/n) 
In addition to the spike, these variants have accumulated a number of mutations in other viral genes - important for viral transmission and immune evasion by the variants. This thread will only focus on the spike because that is what is used in mRNA vaccines. (11/n) 
This 🏳️🌈 graph depicts the PRNT50 of antibodies induced in mRNA vaccinees against the ancestral vs. the 18 variants using authentic viral neutralization assays. You can see the various amino acid changes in the Spike correlating with reduction in NAb capacity. (12/n) 

A linear mixed model by @jessroth95 revealed that 8 of the 11 key S gene mutations had significant negative effects on neutralization, and that L452R and E484K/Q had the greatest individual effects.(13/n) 

ΔH69/V70 & E484K combination was synergistic (decreased neutralization more than the added effects; β=-0.182; p=0.005), L452R & P681R was antagonistic (decreased neutralization less than the added effects; β=0.228; p=0.003), and E484K & N501Y was neither (β=0.060; p=0.248). (14/)
Previously infected people had higher neutralization against variants than the uninfected in response to vaccination. Future vaccine boosters may help to overcome such NAb reduction observed for the variants with the L452R (B.1.617.2) or E484K & N501Y combo (P.1 , B.1.351). (15/) 

Our study is a result of heroic efforts of many people, led by @carolilucas @VogelsChantal @InciYildirim11. Special thanks to Yale SARS-CoV-2 Genomic Surveillance Initiative, and the HCW volunteers for donating blood. @SaadOmer3 @NathanGrubaugh & I co-supervised this work. (End) 

Adding this awesome thread by @NathanGrubaugh to accompany mine above. Much more deeper dive into the viral genetics, mutation comparison, community spread, and implications on vaccination strategies.
https://twitter.com/nathangrubaugh/status/1417178208496197642
• • •
Missing some Tweet in this thread? You can try to
force a refresh