ICU stories: Middle-aged pt with cirrhosis presented to the ED with abd pain and underwent Hartmann's procedure (colectomy - end-colostomy). Next am, pt was hypotensive on rising levo gtt (0.24 from 0.1) and ⬆️lactate (3.4 -> 6.7). S/he was positive 8 liters in 12 hours 😱
After reading the chart, I was almost certain that pt would be congested/fluid intolerant (after 8 liters+ fluid balance...). When I first walked in the room, BP was 90-100/30-40 (radial a-line), HR 120-130 and this is what the monitor showed:
I did POCUS: hyperdynamic LV, no pericardial effusion, RV OK, IVC very small (images not shown). I threw some color Doppler in the LV and this happened:
"A lot of color"! -> high velocity signals in the LV cavity and probably the LVOT. US windows not good (pt on the vent; subcostal views impossible given recent laparotomy), so I tried some continuous Doppler in the LVOT: 

☝️Voila! A dagger-shaped signal with max velocity of 6 m/sec. I was not interested in finding exactly where the obstruction was. I bolused ivf, started vasopressin 0.04 and gave 5 mg iv metoprolol. In a few hours, lactate was normal and levo was ⬇️by 2/3.
Patient seemed to benefit from being managed as one with LV outflow tract obstruction. I would have never tried iv metoprolol in a pt on industrial doses of pressors. In the past, I might have tried esmolol that can be dc/ed fast. POCUS makes us smarter at the bedside. To be fair
the initial monitor view gave me pretty much the diagnosis or at least raised my suspicion of it. Do you see how weird it looks? Do you see the 2 systolic inflections? 

Let's look closer. The obstruction to the ejection of blood from the left ventricle creates the 2nd systolic peak 

This is what happened after fluids were given and heart rate was controlled. The dynamic obstruction to ejection of blood from the left ventricle disappeared
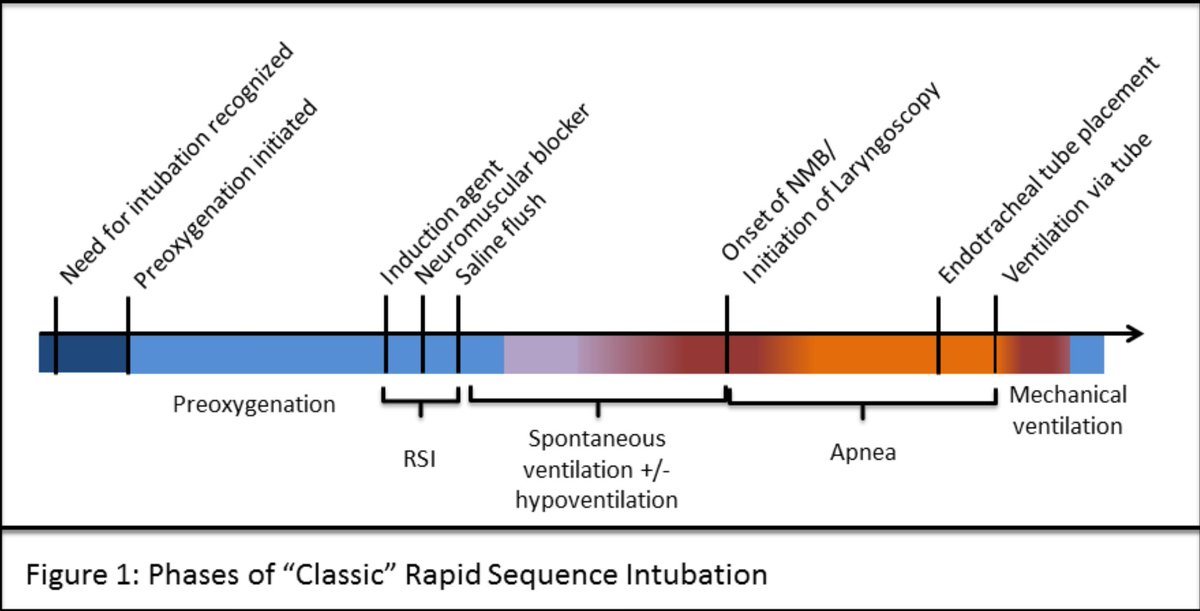
Take home messages:
1. Looking at the arterial/CVP waveforms can be sometimes very helpful, in fact more helpful than the exact BP/CVP values
2. We should not automatically equate a positive fluid balance, even a significant one, with fluid intolerance or venous congestion
1. Looking at the arterial/CVP waveforms can be sometimes very helpful, in fact more helpful than the exact BP/CVP values
2. We should not automatically equate a positive fluid balance, even a significant one, with fluid intolerance or venous congestion
3. We should always consider LV outflow tract obstruction in hemodynamically unstable patients
DOI 10.1007/s12630-009-9174-y
Thanks for reading!
DOI 10.1007/s12630-009-9174-y
Thanks for reading!
• • •
Missing some Tweet in this thread? You can try to
force a refresh