I am very excited to introduce Interleukin-11 signaling as a global regulator of regeneration @ScienceAdvances! Huge thanks to @ReischauerS’s supervision and the co-authors for their support!
#regeneration #scarring #zebrafish
A thread. 1/n
#regeneration #scarring #zebrafish
A thread. 1/n

Regeneration and scarring are the opposing endpoints after tissue damage. Mammals predominantly mount a fibrotic response, a root cause of many diseases. In contrast, cells in regenerative species undergo reprogramming, initiating a regenerative gene program. 2/n
We started by asking how quantitatively different these responses are. After cardiac injury, by lineage tracing fibroblasts and endothelial cells, we found ~10x less scar-forming myofibroblasts in the zebrafish heart when compared to what is known in adult mice. 3/n 

These data suggested that zebrafish potentially activate mechanisms to limit excessive fibrosis. To identify these mechanisms, we profiled regenerating vs. exercised fish hearts. This hunt paved the way to identify Il-6 family-mediated Stat3 signaling as our prime candidate. 4/n 
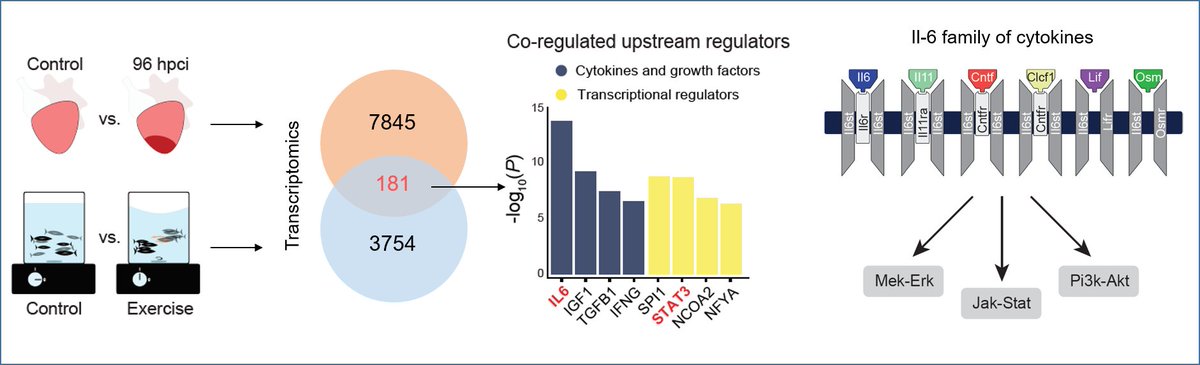
Next, using LOF alleles, we observed that both il6st (the common co-receptor of Il-6 family cytokines) and stat3 zebrafish mutants displayed a similar, dramatic loss of regenerative potential, confirming the pro-regenerative role of Il-6 family-mediated Stat3 signaling. 5/n 

We then tested for the most induced Il-6 family cytokines after injury. Turned out that both paralogs of Il-11 encoding genes were not only rapidly induced in heart and fins, but also that this induction is conserved in regenerative species (axolotl, lungfish, killifish..)! 6/n 

We were very curious to see what Il-11 has to offer, given its debated role in mammalian fibrosis! We mutated the Il-11 receptor and ligands. These zf mutants are viable and fertile, but display severely impaired regeneration in all tissues and at developmental stages tested. 7/n 

Things started heating up! We then asked – what happens downstream of Il-11 signaling that makes it a global regulator of regeneration? Now, this is a two-step answer. 8/n
First – using transcriptomics on hearts and fins, we observed that Il-11 acts upstream of a majority of the global- and tissue-specific regeneration program genes identified to date! 9/n 
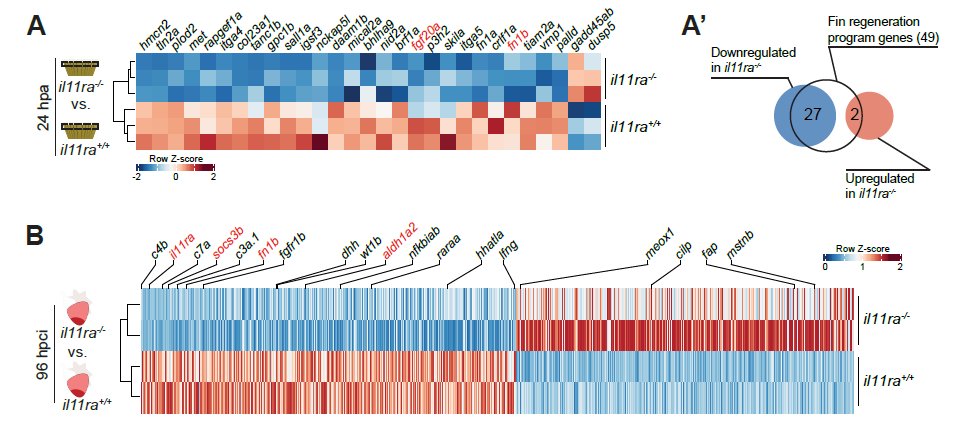
Consequently, we observed a severely compromised repopulation of the injured areas by regenerating cells in fins and hearts – hence, no significant formation of a regenerate. 10/n 

Second – we observed a global activation of hallmarks of mammalian fibrosis, including excessive ECM deposition, myofibroblast differentiation and persistence – in both heart and fins of the non-regenerative il11ra mutants. 11/n 

We knew that we were on the right track when almost every single inducer of mammalian fibrosis, including the recently discovered Meox1, was upregulated in the mutant transcriptome after injury! 12/n
Next, we dove into the cellular and molecular aspects of how Il-11 limits mammalian-like fibrosis – myofibroblast differentiation in the heart, in specific. We observed that il11ra is highly expressed in cardiac endothelial cells. 13/n
We found that, after injury, cardiac endothelial cells in il11ra mutants were hyper-invasive and disorganized compared to the wild types. These data raised the possibility of EndoMT – a phenotype of endothelial cells adopting myofibroblast-like characteristics! 14/n
Indeed, lineage tracing endothelial cells confirmed that Il-11 signaling limits EndoMT after cardiac injury in zebrafish. As a side note – il11ra is also expressed in cardiac fibroblasts, and limits myofibroblast fate in this cell type during regeneration as well. 15/n 

This means that Il-11 signaling is promoting the activation of a regenerative gene program in cells of the heart and fins, a process that we describe as regenerative reprogramming. Therefore, when Il-11 is not present, these cells instead adopt a scar-forming phenotype. 16/n
Now, the question that I like a lot – does Il-11 signaling in endothelial cells talk to cardiomyocytes? You guessed it right! It does! Endothelial-specific re-expression of il11ra in il11ra mutants not only rescued endothelial defects, but also cardiomyocyte migration. 17/n 

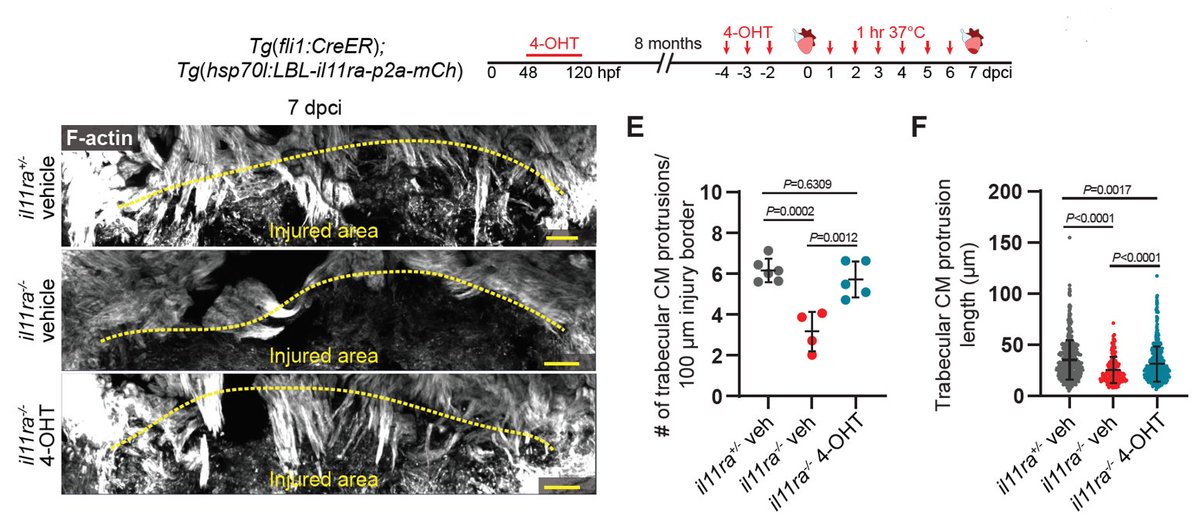

Overall, we identify Il-11 signaling as the first global regulator of regeneration that promotes cellular reprogramming and limits mammalian-like scarring. Check out the paper for more details and a bonus feedback loop between Il-11 and TGFB axes.
tinyurl.com/4fhd2kdb 18/n
tinyurl.com/4fhd2kdb 18/n
Huge thanks again to all of our collaborators, funding sources, Didier for his constant support, @_Priya_R, @rmarinjuez, and @BensimonBrito for discussions. Thanks to everyone @mpihlr @jlugiessen. Finally, thanks to @ALJoyner21 and the reviewers for pushing the boundaries! 19/n
Looking forward, our zebrafish data, with the contradicting mammalian work hint that the secrets of regeneration lie in the differences downstream of Il-11 signaling between regenerative and non-regenerative species.
End of the thread, but the beginning of more exciting science!
End of the thread, but the beginning of more exciting science!
• • •
Missing some Tweet in this thread? You can try to
force a refresh




