Have you heard of the recently discovered 8th human-infecting #coronavirus designated CCoV-HuPn-2018?
We reveal the architecture of its spike (ie infection machinery), receptor usage and antigenic properties!
Led by @aletortorici
1/12
biorxiv.org/content/10.110…
We reveal the architecture of its spike (ie infection machinery), receptor usage and antigenic properties!
Led by @aletortorici
1/12
biorxiv.org/content/10.110…
CCoV-HuPn-2018 is a canine-feline recombinant alpha-#coronavirus isolated from the respiratory swab of a child hospitalized with pneumonia, indicating that more coronaviruses are spilling over to humans than previously appreciated.
2/12
academic.oup.com/cid/advance-ar…
2/12
academic.oup.com/cid/advance-ar…
We determined #cryoEM structures of the CCoV-HuPn-2018 spike in two markedly different conformational states which we propose to correspond to two snapshots of viral entry.
3/12
3/12

The CCoV-HuPn-2018 spike harbors a domain 0 upstream from the 'canonical NTD' (aka domain A) and they share the same fold (despite <13% aa sequence identity). Domain 0 interacts with host carbohydrates for some alphacoronaviruses and perhaps CCoV-HuPn-2018 does too.
4/12
4/12

Based on structural similarity with PRCV/TGEV, we postulated that the CCoV-HuPn-2018 spike receptor-binding domain (RBD, domain B) could bind APN which is an entry receptor for some alphacoronaviruses, including PRCV/TGEV.
5/12
5/12

The CCoV-HuPn-2018 spike receptor-binding domain (RBD, domain B) binds to feline, canine & porcine APN orthologs but not human APN (similar to PRCV/TGEV), which is surprising as CCoV-HuPn-2018 was isolated from an hospitalized child.
6/12
6/12

It turns out that one of the differences between human APN and feline, canine & porcine APNs is a Thr to Arg residue substitution at position 741, in the receptor region recognized by PRCV/TGEV.
7/12
7/12

Previous work showed that abrogation of this glycosylation site prevented binding of the TGEV RBD to cell-surface expressed porcine APN and we wondered if it could be the case for CCoV-HuPn-2018 as well.
8/12
journals.plos.org/plospathogens/…
8/12
journals.plos.org/plospathogens/…
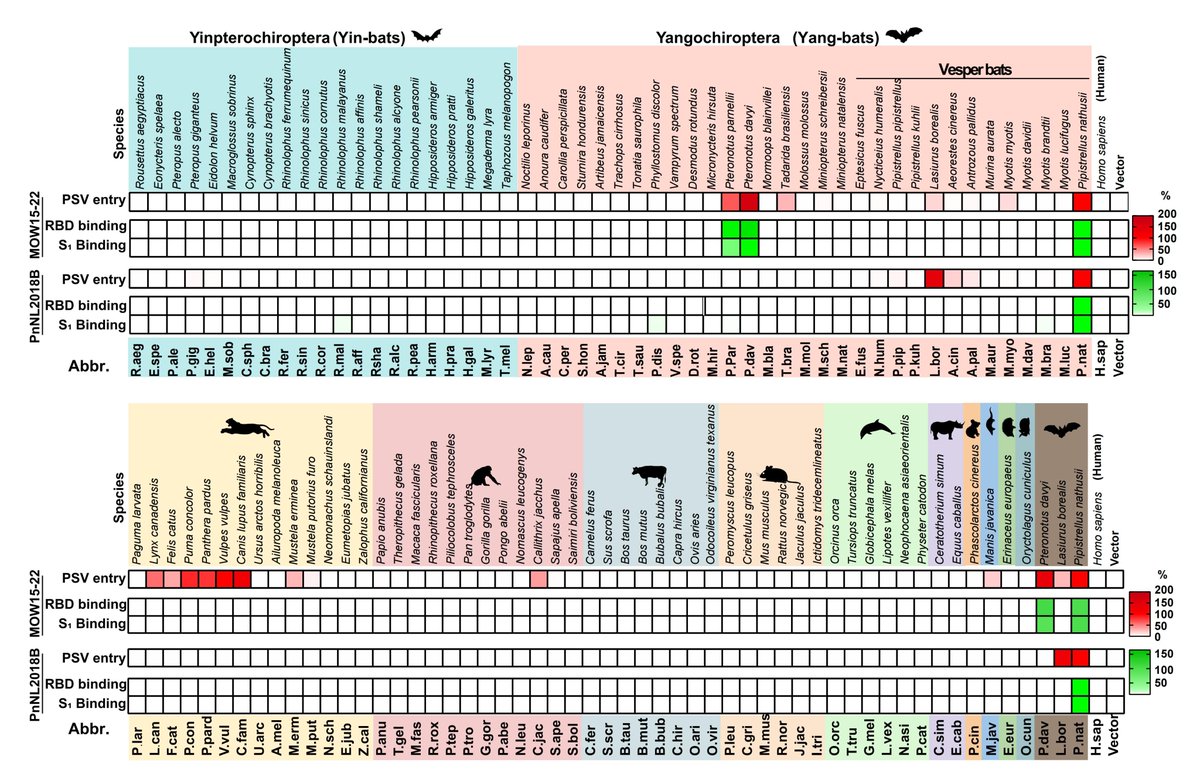
Feline, canine & porcine APNs, and human R741T APN (N739 oligosaccharide knockin mutant) render cells permissive to CCoV-HuPn-2018 spike-mediated entry and soluble APNs inhibit this in a concentration-dependent manner. Thus APN is an entry receptor for CCoV-HuPn-2018!
9/12
9/12

APN single nucleotide polymorphisms have been described in humans, including at R741 (wo introducing a glycan) and we think that such variations might be present in infected individuals.
Please get in touch if interested in helping us find out.
10/12
sciencedirect.com/science/articl…
Please get in touch if interested in helping us find out.
10/12
sciencedirect.com/science/articl…
Polyclonal plasma neutralizing antibodies elicited by 'common cold' 229E infection also cross-neutralized (to various extent) CCoV-HuPn-2018 spike-mediated entry, which has the potential to reduce disease severity of this virus in humans.
11/12
11/12

This work would not have been possible without @coronalexington Anshu Joshi @YoungjunPark11 and our fantastic collaborators @atelentia @LanzavecchiaB @DavideCorti6 @jbloom_lab and many others not on twitter
12/12
12/12
• • •
Missing some Tweet in this thread? You can try to
force a refresh