27 November:Temel, QoL outcomes
This year for Lung Cancer Awareness Month #LCAM I’m going to summarize 30 important lung cancer trials over 30 days. These posts are directed at non-medical professionals, with descriptions of the results and of what makes a good trial.#lcsm 1/19
This year for Lung Cancer Awareness Month #LCAM I’m going to summarize 30 important lung cancer trials over 30 days. These posts are directed at non-medical professionals, with descriptions of the results and of what makes a good trial.#lcsm 1/19

Today’s trial is one of the most thought-provoking of the month, and it has been discussed widely since its publication in 2010. It is a trial looking at the timing of referral to palliative care for people with advanced, incurable lung cancer. 2/19
Many people hold the view that palliative care is care at the end of life. While this is a component of it, palliative care physicians are experts in controlling symptoms, which is valuable in a highly-symptomatic disease like metastatic lung cancer. 3/19
This trial enrolled 151 people at Massachusetts General Hospital between 2006-2009. They were randomized to be referred to a palliative care specialist either within 12 weeks of diagnosis, or at the discretion of the oncologist (standard care). 4/19
The primary outcome was a Quality of Life measure, taken 12 weeks after diagnosis. The FACT-L (Functional Assessment of Cancer Therapy-Lung) is a questionnaire where respondents rate 36 items from 0-4, which are then summed to a total score (TOI). Page 1 is reproduced below. 5/19 

There were numerous other outcomes, including additional questionnaires focused on mood and depression, type and place of end of life care, and survival. 6/19
The trial was positive, with TOI improving by 2.3 points in the palliative care group, and deteriorating by 2.3 points in the standard of care group (p=0.04). People in the palliative care group had better depression scores, but no difference in anxiety scores. 7/19 

Provocatively, patients in the early palliative care group were less likely to receive aggressive end of life care, defined as any of:
1. Chemotherapy within 14 days of death
2. Never admitted to hospice
3. Admitted to hospice within 3 days of death
8/19
1. Chemotherapy within 14 days of death
2. Never admitted to hospice
3. Admitted to hospice within 3 days of death
8/19
Most provocative of all, despite having less aggressive therapy at the end of life, people in the early palliative care group lived longer (median OS 11.6 vs 8.9 months, p=0.02). 9/19 
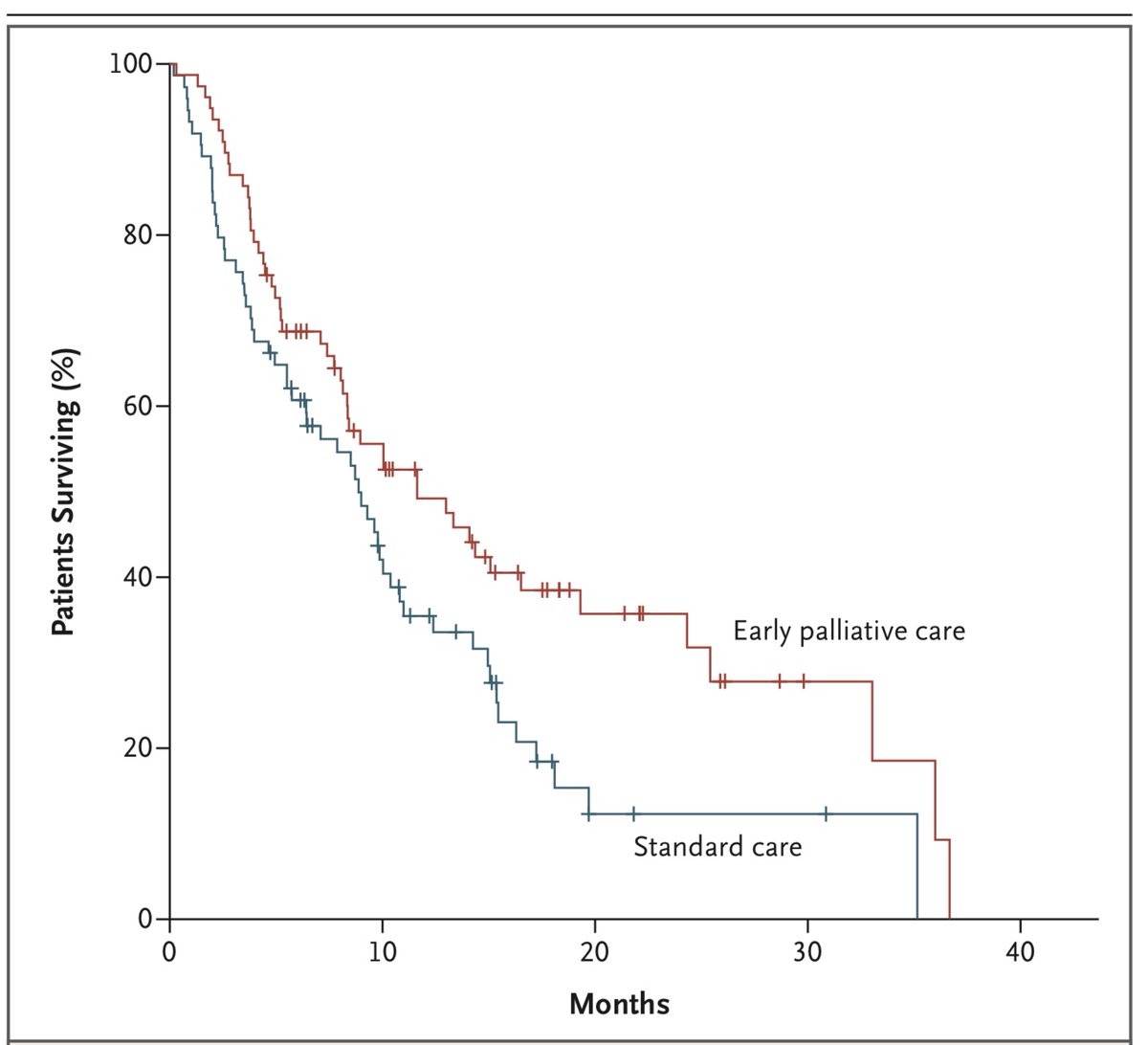
I want to talk about QoL outcomes before discussing the issues raised by this trial. There is widespread agreement that Quality of Life is a crucial outcome for patients, as important (or more so) than duration of survival. But how to measure it? 10/19
Most studies use a measure like this trial did: a composite score of one or many questionnaires about symptoms and experiences. They are administered sequentially, and usually report changes over time, rather than scores at a single time point. 11/19
Many oncologists feel some unease about these endpoints:
1. There are several competing questionnaires
2. The outcomes are mathematically complex, subject to potential manipulation
3. QoL seems ineffable: to these questionnaires truly capture it?
12/19
1. There are several competing questionnaires
2. The outcomes are mathematically complex, subject to potential manipulation
3. QoL seems ineffable: to these questionnaires truly capture it?
12/19
This trial has inspired a lot of commentary: firstly, is the Overall Survival advantage real? Certainly, it was a surprising finding, and likely the reason the trial was so widely read. It was a secondary endpoint, so not included in the 5% false-positive tolerance of the study.
Subsequent studies have also hinted at a survival advantage with quality palliative care. There is probably a real advantage there, but I think that even if there was not, the other advantages of early palliative care are meaningful, and are what we should want for our patients.
Second: as an oncologist, how much of this care can I provide myself? Though we focus on provision of active treatments, asking about symptoms, coping, and mood ought not to be outside our scope of practice, even if we are supported by palliative care specialists. 15/19
Third, the combination of less intense cancer care at end of life and improved survival has made me reflect on whether later lines of therapy actually make things worse.
There are lots of motivations for both patients & doctors to continue treatment into 3rd, 4th, 5th etc lines.
There are lots of motivations for both patients & doctors to continue treatment into 3rd, 4th, 5th etc lines.
For some people this makes sense. For many though, particularly as performance status starts to decline, there likely comes a time where more cancer treatments are not just futile, but actually harmful. 17/19
I think this is a common problem. A few years ago in my clinic a resident asked me how many lines of therapy patients got, on average.
My immediate and unthinking response: “One too many”.
18/19
My immediate and unthinking response: “One too many”.
18/19
To date these reviews have focused almost exclusively on phase III randomized controlled trials. The next two days will look at non-randomized trials, and at randomized phase II designs, to discuss their strengths and weaknesses. 19/19 

• • •
Missing some Tweet in this thread? You can try to
force a refresh