Our first initial estimates of vaccine effectiveness (VE) against symptomatic disease with the Omicron variant are now out. In short, VE remains high following a Pfizer booster after AZ or Pfizer, but is reduced after two doses.
More below 👇
1/11
More below 👇
1/11
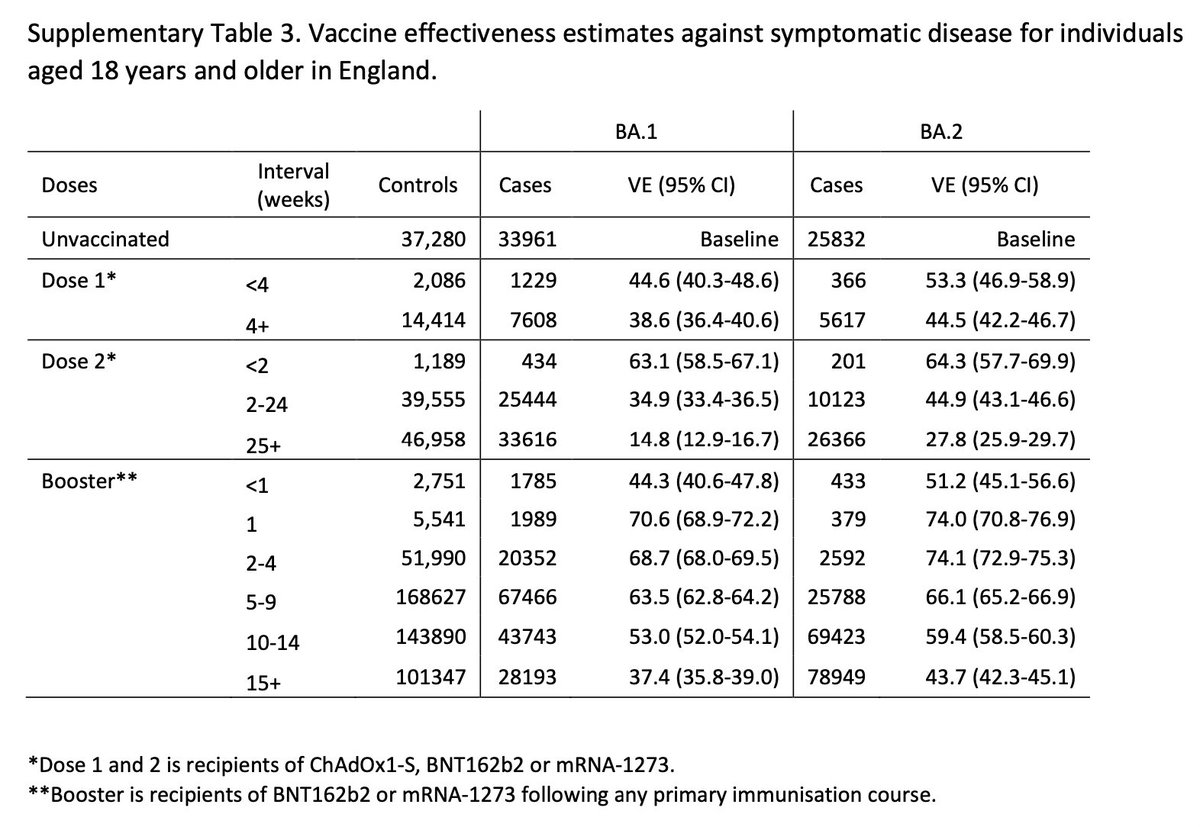
We used a test negative case control design to estimate VE against symptomatic COVID-19 with the Omicron variant compared to Delta. The odds of vaccination in PCR positive cases was compared to the odds of vaccination in those who test negative.
2/11
2/11
Pillar 2 tests were classified as either Delta or Omicron from the period 27/11 – 6/12 based on sequencing and SGFT where sequencing wasn’t available. From 27/11, at least 80% of PCR tests which included the S-gene as a target and which had SGTF were the Omicron variant.
3/11
3/11
581 symptomatic Omicron cases were identified during the study period which could be linked to the vaccine record. Over the same period there were 56,439 eligible Delta cases and 130,867 test negative controls.
4/11
4/11
Among those who received AZ as the primary course, from two weeks after a Pfizer booster, VE increased to 71.4% (95%CI: 41.8 to 86.0%). Among those who had received Pfizer as the primary course, VE increased to 75.5% (95%CI: 56.1 to 86.3%) after the booster.
5/11
5/11
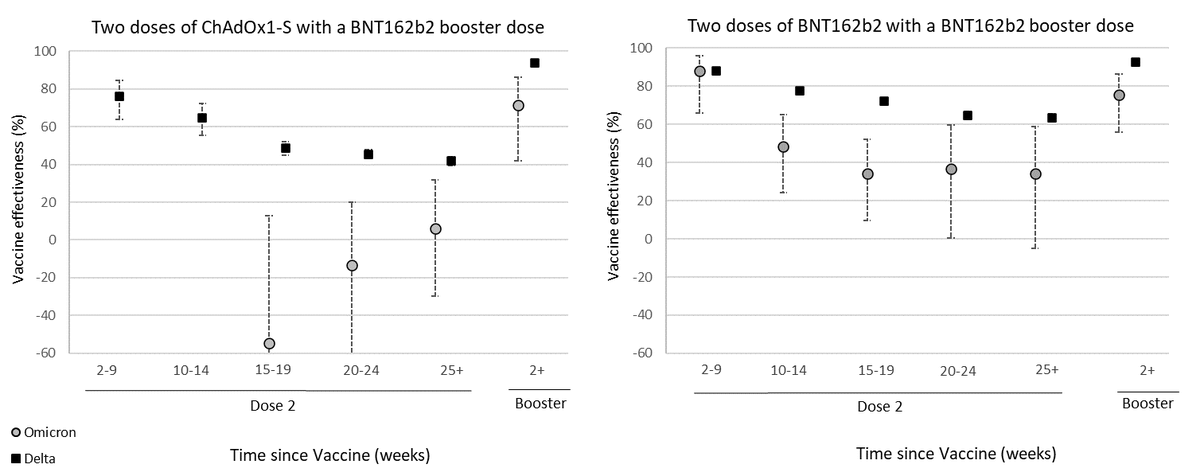
Our findings show that VE against symptomatic disease with the Omicron variant is lower than with the Delta variant, although moderate to high VE of 70-75% is seen in the early period after a booster.
6/11
6/11
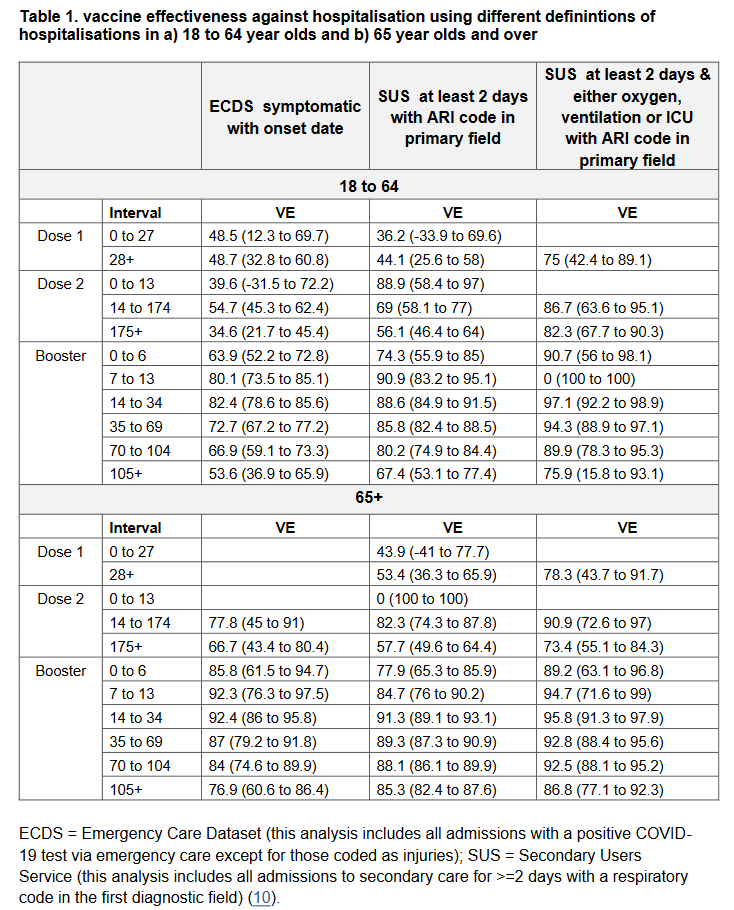
Due to the small numbers of Omicron cases detected and the time-lag between infection and severe disease, we have not yet been able to determine how protective the vaccines are against severe outcomes.
7/11
7/11
However - previous experience with the Delta variant suggests that protection against hospitalisation after two doses is well maintained. We will be looking at this as soon as there is enough data!
8/11
8/11
These findings are based on small numbers of cases with the Omicron variant and our estimates are subject to significant uncertainty with wide confidence intervals. This will improve as more data become available.
9/11
9/11
Our findings support maximising coverage with booster doses in highly vaccinated populations. Further follow-up will be needed to assess VE against more severe outcomes (such as hospitalisation) as well as the duration of protection of the booster vaccine.
10/11
10/11
• • •
Missing some Tweet in this thread? You can try to
force a refresh