~Moved to Blue Sky~
Senior epidemiologist - respiratory virus surveillance and vaccine evaluations @UKHSA
5 subscribers
How to get URL link on X (Twitter) App




 Here, we find no reduction in VE against symptomatic disease for BA.2 as compared to BA.1.
Here, we find no reduction in VE against symptomatic disease for BA.2 as compared to BA.1. 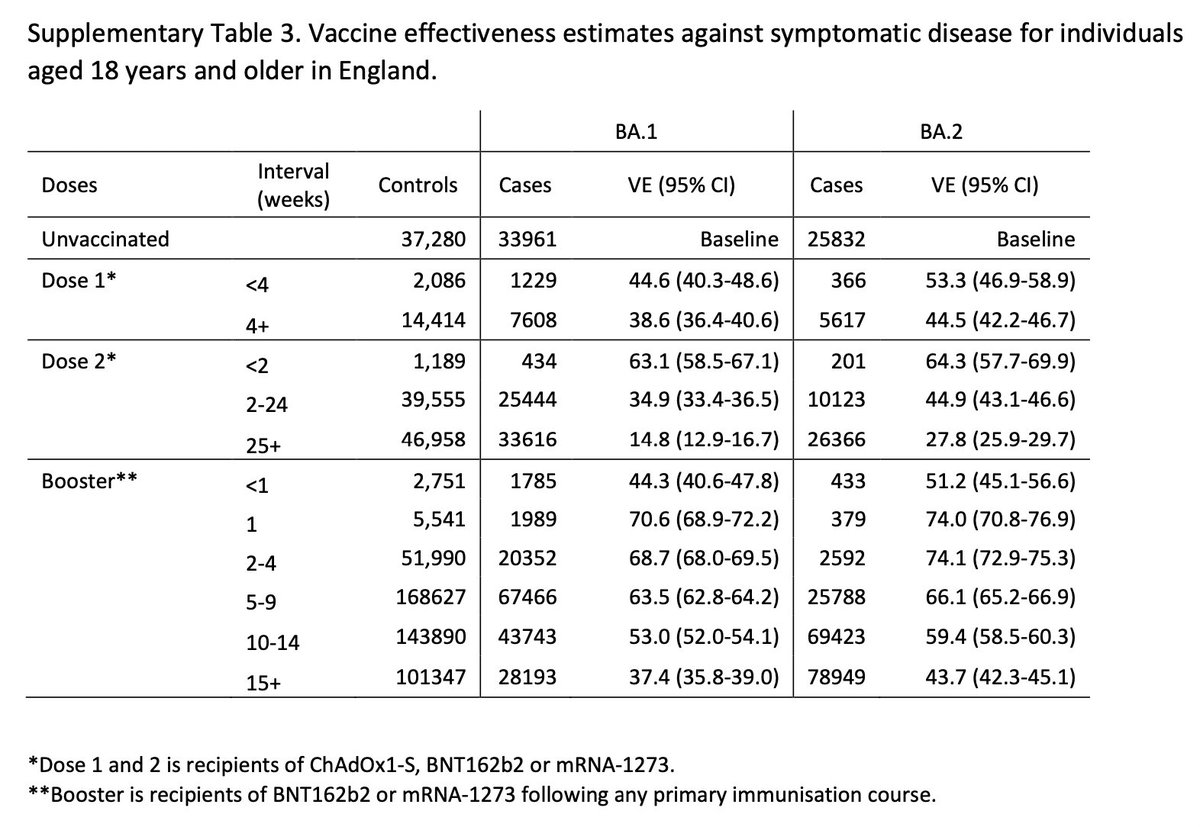

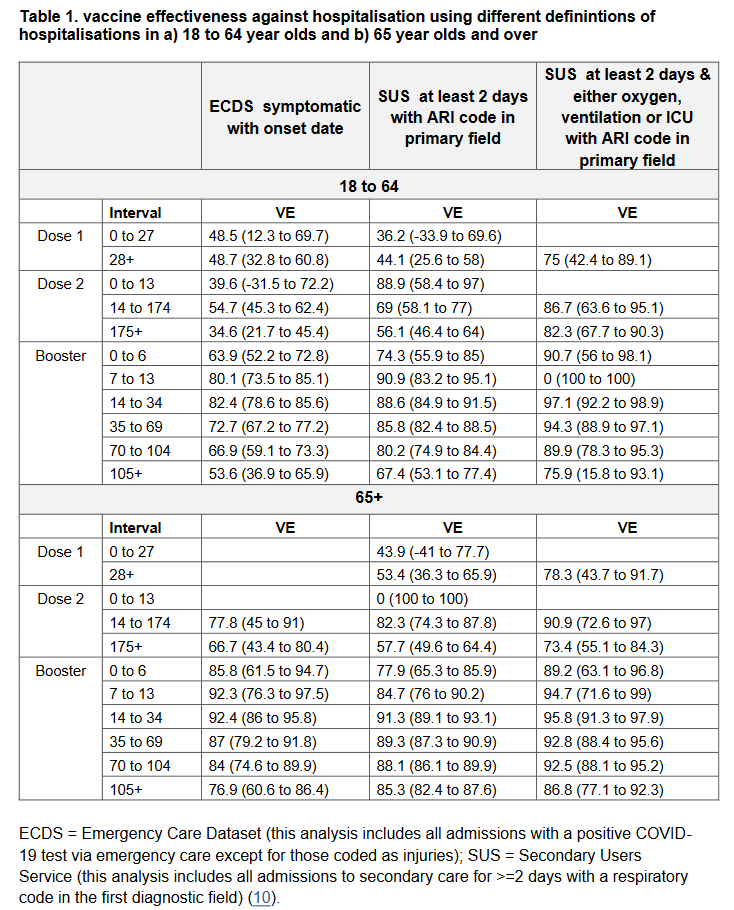
 Since Omicron causes milder disease and all individuals who are hospitalised for any reason in the UK are tested for COVID-19, an increasing proportion of cases in hospital are likely to have COVID-19 as an incidental finding rather than as the primary reason for admission.
Since Omicron causes milder disease and all individuals who are hospitalised for any reason in the UK are tested for COVID-19, an increasing proportion of cases in hospital are likely to have COVID-19 as an incidental finding rather than as the primary reason for admission.


https://twitter.com/UKHSA/status/1479526192428503044