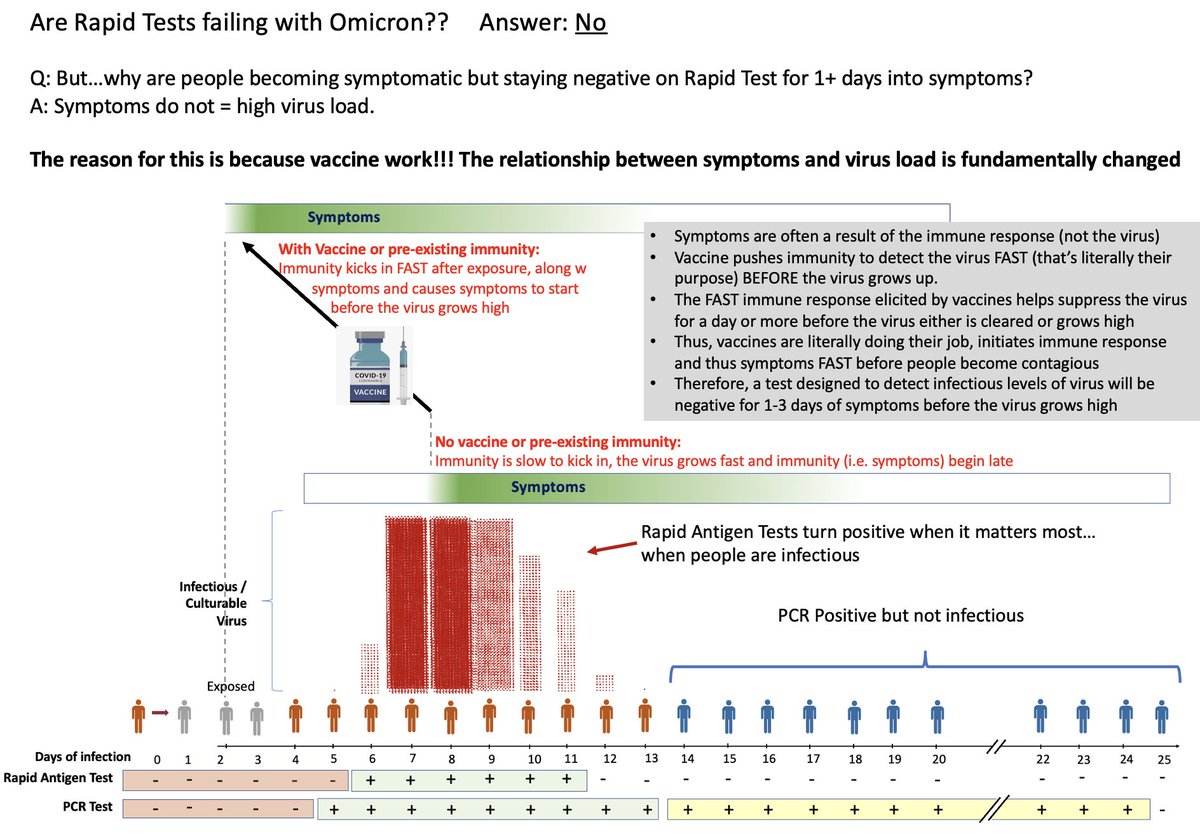
REMINDER
Somewhere over the year the importance of FREQUENCY of tests got lost
NO test is perfect
"Test Sensitivity" is MUCH MORE about frequency
If test fails to turn pos on day 1, check day 2. If pos day 2, you can stop transmission days 2,3,4,5,6
nejm.org/doi/full/10.10…
Somewhere over the year the importance of FREQUENCY of tests got lost
NO test is perfect
"Test Sensitivity" is MUCH MORE about frequency
If test fails to turn pos on day 1, check day 2. If pos day 2, you can stop transmission days 2,3,4,5,6
nejm.org/doi/full/10.10…
And yes. I know tests are $$ / unavailable
Believe me, I’ve tried probably more than any human on earth (literally) to have us not arrive where we are:
with a predicted variant overtaking vaccines in a world where no one has access to tests
I am sorry I couldn’t do more
2/
Believe me, I’ve tried probably more than any human on earth (literally) to have us not arrive where we are:
with a predicted variant overtaking vaccines in a world where no one has access to tests
I am sorry I couldn’t do more
2/
And going back to first tweet. If testing is not frequent then likelihood of testing on day 1,2 is not terribly high so by time you test, you’ll usually be positive if you are infectious. This is how it’s always worked
W Omicron it raises stakes further for speed/frequency
3/
W Omicron it raises stakes further for speed/frequency
3/
And additionally - quarantine if symptomatic. Period. If you are symptomatic and you have any chance of having been exposed (ie most humans) assume Omicron and quarantine if possible.
4/
4/
• • •
Missing some Tweet in this thread? You can try to
force a refresh