🧵 on the physiology of regional strain. While the added value of global longitudinal strain is doubtful, compared to MAPSE, strain and strain rate was, and is still a method for visualising inequalities of *Regional* systolic shortening.
1/ B-mode provides all the necessary information where changes are large and obvious, as in this case, but regional strain/strain rate may be of added value in giving some added physiological information
2/ in other cases, where changes are small, strain and strain rate may add diagnostic value.
3/ However, regional strain and strain rate are vulnerable to all kinds of noise and other distortions, as well as method and vendor dependent. pubmed.ncbi.nlm.nih.gov/28528162/
4/ But the physiological mechanisms are independent of vendor and method. Qualitative evaluation of curve shape or colour M-mode are thus far more robust, while containing most of the pathophysiological information. This is Strain and strain rate from the septum of pat. in 2/ 

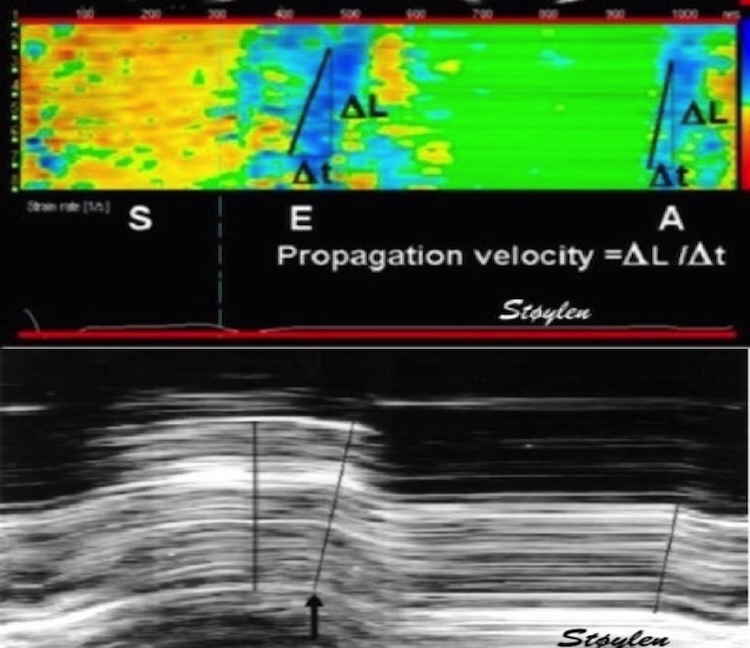
5/ the curve shapes of strain rate and strain gives the same information, but strain may be more intuitive. Colour M-mode is another semi-quantitative approach, giving much of the same information, useful for evaluating timing as well as extent of pathology. 

6/ Even with different technology, speckle tracking and Tissue Doppler gives roughly the same information, relating to the same physiology, as in this example from the case in tweet 1/ 

7/ To understand the physiology of altered regional function, it is necessary to look at shortening versus load. In a symmetric ventricle the load can be described by intracavitary pressure, P, wall thickness, h, and the radius of regional curvature, r by the law of Laplace.
8/ Looking at the experimental model of isolated papillary muscle, it explains this well. As the muscle develops tension (force), the contraction is isometric, until tension = load, where the muscle starts to shorten, in an isotonic contraction. 

Of course, this model is simplified, relating to the whole heart, but is still useful in explaining the physiology. 

9/ The papillary muscle model illustrates the dependence of strain and strain rate on load, in the case of unchanged contractility. pubmed.ncbi.nlm.nih.gov/13978233/ 

10/ Contractility changes also affect strain and strain rate, also with constant load. Contractility change also affects relaxation rate. pubmed.ncbi.nlm.nih.gov/14220048/
In the case of ischemia, the prolongation of relaxation is even more pronounced, as SERCA is energy dependent
In the case of ischemia, the prolongation of relaxation is even more pronounced, as SERCA is energy dependent

In general, the wall stress is fairly equally distributed over the ventricle, curvature being greatest in the apex, but the apical wall is also thinnest. P, of course is the same for all parts of the ventricle. Thus all segments shows approximately the same shortening. 

11/As long as contractility is normal in all segments, this will be the pattern. However, looking at the longitudinal tension, it is also part of the load of the neighbouring segment. If both segments (red and blue) have normal contractility, the tension balances. 

12/ A segment with reduced contractility (blue), will produce less tension. Thus:
1: Shortening of the segment is reduced
2: Shortening of neighbouring segment is increased due to less load.
3: Delayed relaxation in the segment, thus shortening when the normal segment relaxes
1: Shortening of the segment is reduced
2: Shortening of neighbouring segment is increased due to less load.
3: Delayed relaxation in the segment, thus shortening when the normal segment relaxes

13/ With less tension, there is less shortening, until tension is too low to shorten the segment, but instead it will stretch. But with some tension and delayed relaxation, there will still be PSS 



14/ Basically, in regional dysfunction (or asynchrony), the tension of a segment is part of the load of the other segments, and the strain pattern depends on the tension-load-shortening relationship. Looking at the systole: 

15/ In ischemia, global tension is reduced, giving globally reduced annular motion. But load induced regional hyperkinesia in non ischemic segments, gives reduction of annular in all walls. Annular motion cannot locate iskemia.
pubmed.ncbi.nlm.nih.gov/14534065/
pubmed.ncbi.nlm.nih.gov/32600336/
pubmed.ncbi.nlm.nih.gov/14534065/
pubmed.ncbi.nlm.nih.gov/32600336/
16/ - and when infarcted segments recover, the hyperkinesia of healthy segments reverses as well, although, we didn't quite understand the mechanism of the hyperkinesia at that time. pubmed.ncbi.nlm.nih.gov/15891749/ 

• • •
Missing some Tweet in this thread? You can try to
force a refresh