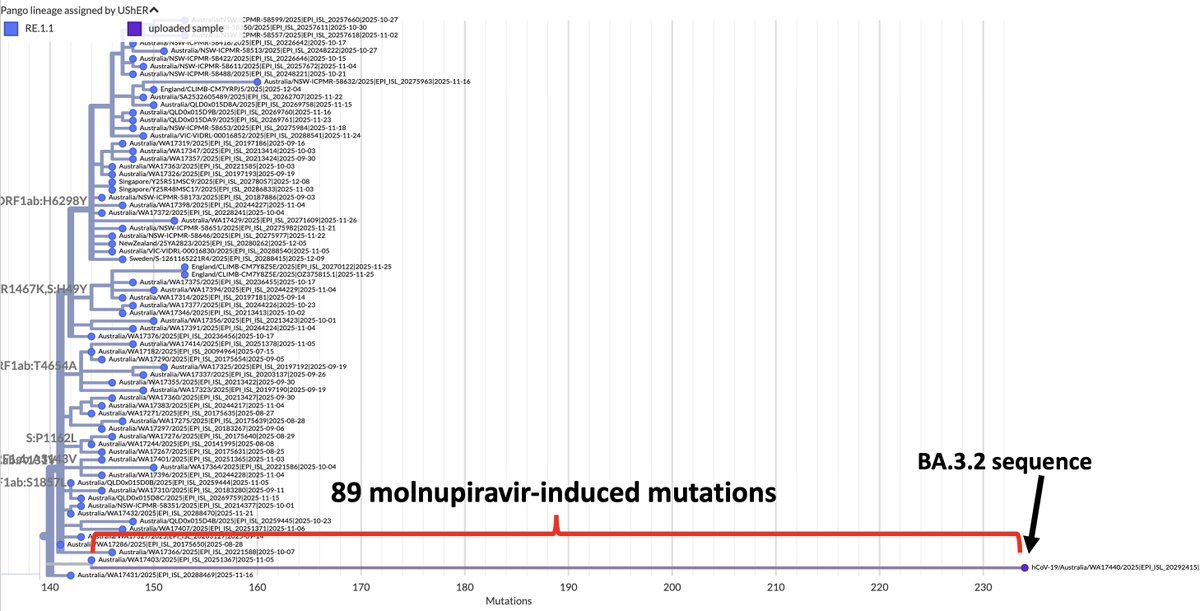
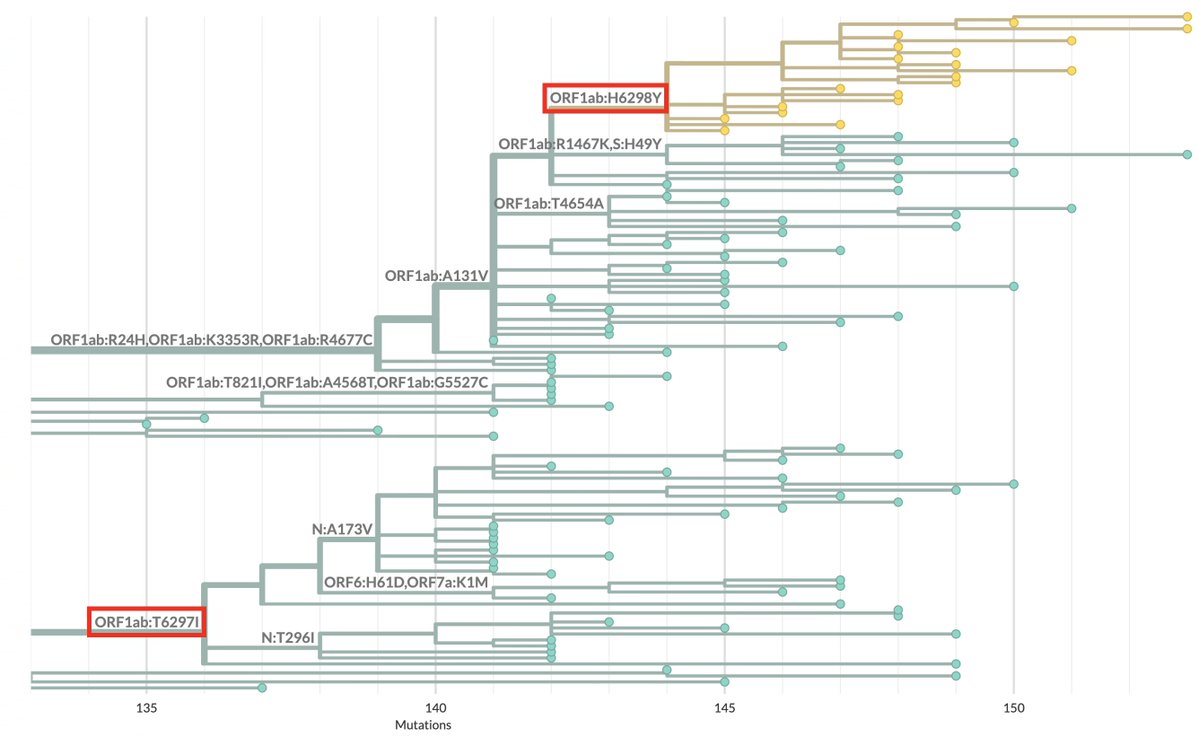
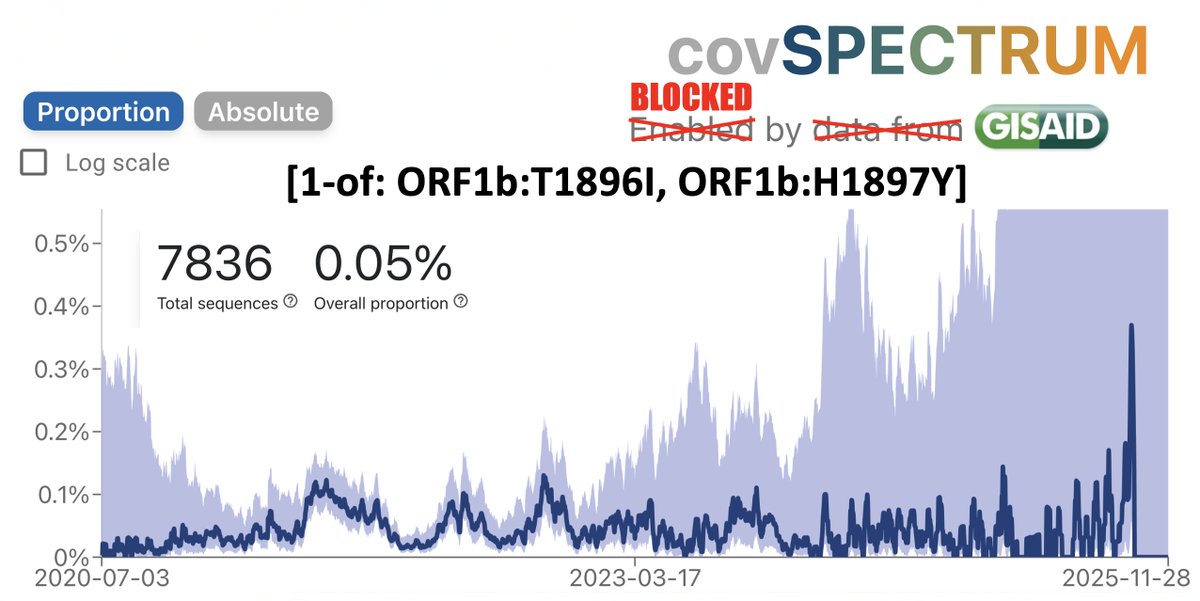
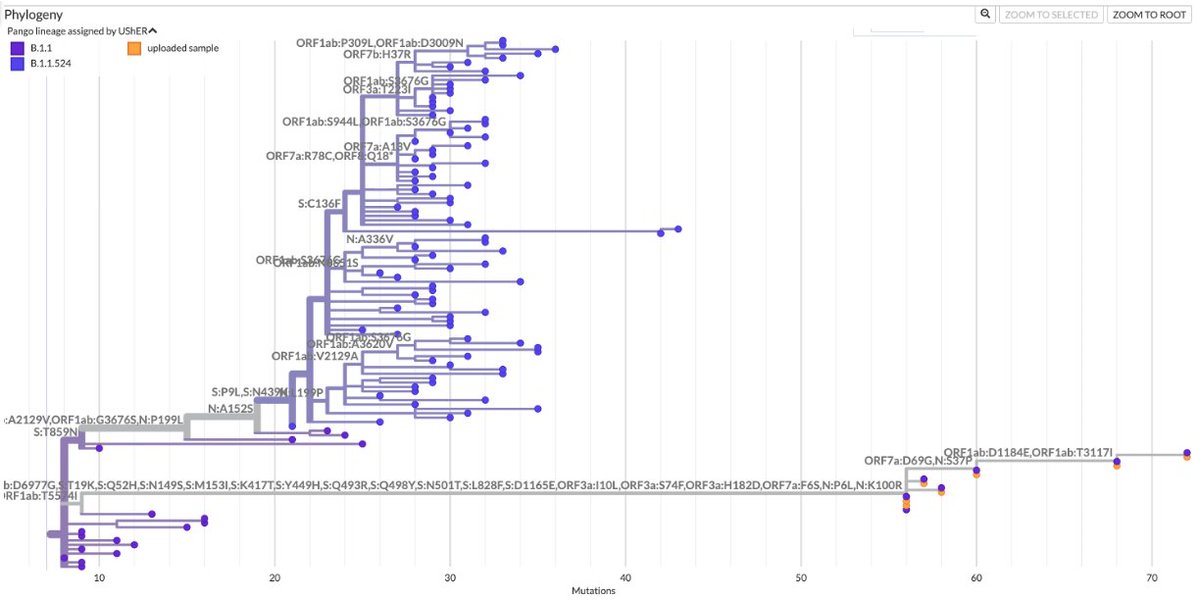
1/6 At a private gathering of 33 Pfizer-triple-vaccinated health care workers in the Faroe Islands, 21 were infected with Omicron—a superspreading event among 3-dose-vaccinated people. All received dose #3 within 2.5 months of the event. Very discouraging.
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…

2/6 All participants tested negative within 36 hours of the event: five with rapid tests and the other 28 with PCR tests. Median age was 45. Only four of the 21 infected had any comorbidities. 



3/6 And very similar to the Oslo Christmas superspreader event (where 79 of 80 infected were symptomatic, with 74 having >3 symptoms), all 21 experienced symptoms. There is virtually no asymptomatic infection with Omicron it seems. 

4/6 While most had mild illness, moderate and severe symptoms were not as rare as one would hope in a group of young, healthy, triple-vaccinated individuals. Thankfully, none were hospitalized. 

5/6 As seen in the Oslo superspreading event and described anecdotally elsewhere, the incubation period was short: 3.24 days on average. Five of those infected still had symptoms at the time of their interview (12-14 days after infection). 

6/6 CDC messaging has consistently downplayed the risk of transmission among the vaccinated, & it needs to stop. These people did everything right: they were triple-vaxxed & all tested before gathering. It didn't matter.
https://twitter.com/BillHanage/status/1474422534439424010
• • •
Missing some Tweet in this thread? You can try to
force a refresh