1/ SUPERSPREADING IS EXPLAINED BY SHARED-ROOM AIRBORNE TRANSMISSION
We can use that to estimate risk of transmission for omicron & other diseases
Our peer-reviewed paper was just published (open access) in @EnvSciTech
I'll explain it in this thread
pubs.acs.org/doi/abs/10.102…
We can use that to estimate risk of transmission for omicron & other diseases
Our peer-reviewed paper was just published (open access) in @EnvSciTech
I'll explain it in this thread
pubs.acs.org/doi/abs/10.102…
2/ Some background first, for people who may not have seen this before:
We have known for a long time that airborne transmission is dominant for COVID-19. See this thread for some of the evidence base:
We have known for a long time that airborne transmission is dominant for COVID-19. See this thread for some of the evidence base:
https://twitter.com/jljcolorado/status/1383566908797059078
3/ The fact that airborne transmission is important is widely accepted in the scientific community
[except for some recalcitrant holdouts in IPC (infection prevention and control)]
But e.g. @WHO admits it clearly on its webpage:
who.int/news-room/ques…
[except for some recalcitrant holdouts in IPC (infection prevention and control)]
But e.g. @WHO admits it clearly on its webpage:
who.int/news-room/ques…

4/ There are 3 general situations for airborne transmission:
a) close proximity -- very dangerous, can inhale a lot of exhaled breath from someone else w/ virus-containing aerosols
b) shared room-air: people in the same room for a period of time, but not very close
a) close proximity -- very dangerous, can inhale a lot of exhaled breath from someone else w/ virus-containing aerosols
b) shared room-air: people in the same room for a period of time, but not very close

5/ (cont. of situations for airborne transmission)
c) longer-range transmission: transmission when not in the same room
[Note that often scientists lump b & c under "long-range transmission. In this paper we separate b vs. c and argue it is useful to do so]
c) longer-range transmission: transmission when not in the same room
[Note that often scientists lump b & c under "long-range transmission. In this paper we separate b vs. c and argue it is useful to do so]
6/ The paper focuses on situation (b), shared room transmission
Before explaining it, few things about (a) & (c):
(a) close proximity transmission is very common, lots of COVID cases. It has been confused w/"large droplet trans." due to historical error:
Before explaining it, few things about (a) & (c):
(a) close proximity transmission is very common, lots of COVID cases. It has been confused w/"large droplet trans." due to historical error:
https://twitter.com/jljcolorado/status/1391111720526024708
7/ Situation (c) "longer-range transmission", when not in the same room, is possible and there are many reported cases for COVID-19. Some examples:
- Transmission in quarantine hotel in New Zealand, most likely due to flow under doors:
wwwnc.cdc.gov/eid/article/28…
- Transmission in quarantine hotel in New Zealand, most likely due to flow under doors:
wwwnc.cdc.gov/eid/article/28…

8/ Another case of (c) longer-range transmission beyond a shared room. This time in quarantine hotel in Hong Kong.
[Very clear cases, bc cameras, very controlled movements, genomic match of virus, few cases in community]
Suspect flow under doors as well
wwwnc.cdc.gov/eid/article/28…
[Very clear cases, bc cameras, very controlled movements, genomic match of virus, few cases in community]
Suspect flow under doors as well
wwwnc.cdc.gov/eid/article/28…
9/ Another case of (c) longer-range transmission beyond a shared room: transmission through air connections in an apartment building in Korea
[Other similar cases in e.g. China]
ijidonline.com/article/S1201-…
[Other similar cases in e.g. China]
ijidonline.com/article/S1201-…

10/ DILUTION is key:
(a) close proximity: LOW dilution, lots of transmission
(b) shared room: LOW dilution IF low ventilation: then superspreading events
(c) longer range (not same room): HIGHER dilution typically, so more difficult (unless unlucky air path w/ low dilution)
(a) close proximity: LOW dilution, lots of transmission
(b) shared room: LOW dilution IF low ventilation: then superspreading events
(c) longer range (not same room): HIGHER dilution typically, so more difficult (unless unlucky air path w/ low dilution)

11/ Back to the paper: many SUPERSPREADING events for COVID, one person infects many, always SHARING A ROOM (case b)
We know superspreading is important overall, as ~5-20% of infected result in ~80-90% of new cases
nature.com/articles/s4159…
We know superspreading is important overall, as ~5-20% of infected result in ~80-90% of new cases
nature.com/articles/s4159…
12/ Some people argue that contact tracing data shows that (a) close proximity dominates and (b) shared-room air is not very important
But CIRCULAR ARGUMENT: only contact trace those in close proximity for > 15 min. Like drunk person only looking for keys under the streetlight
But CIRCULAR ARGUMENT: only contact trace those in close proximity for > 15 min. Like drunk person only looking for keys under the streetlight
13/ What we do in the paper is to investigate superspreading events from the literature, and see if the fit airborne transmission model. Include:
- Skagit choir: onlinelibrary.wiley.com/doi/10.1111/in…
- Guangzhou restaurant: doi.org/10.1016/j.buil…
- Korean call center: wwwnc.cdc.gov/eid/article/26…
- Skagit choir: onlinelibrary.wiley.com/doi/10.1111/in…
- Guangzhou restaurant: doi.org/10.1016/j.buil…
- Korean call center: wwwnc.cdc.gov/eid/article/26…
14/ More cases included in the paper:
- Long-haul aircraft flight: wwwnc.cdc.gov/eid/article/26…
- German meat plant: embopress.org/doi/full/10.15…
- School in Israel: eurosurveillance.org/content/10.280…
- Chinese bus: sciencedirect.com/science/articl…
- And several more
- Long-haul aircraft flight: wwwnc.cdc.gov/eid/article/26…
- German meat plant: embopress.org/doi/full/10.15…
- School in Israel: eurosurveillance.org/content/10.280…
- Chinese bus: sciencedirect.com/science/articl…
- And several more
15/ We analyze these outbreaks together, with a model of airborne transmission in a shared room. Based on the Wells-Riley model (pioneers who demonstrated tuberculosis as the first airborne disease):
dx.doi.org/10.1164/arrd.1…
dx.doi.org/10.1164/arrd.1…
16/ Here is a schematic of the model:
Step 1) Infected person exhales virus (E in drawing). Low amount if breathing, more if talking, much more if shouting, singing loudly, or exercising
Step 1) Infected person exhales virus (E in drawing). Low amount if breathing, more if talking, much more if shouting, singing loudly, or exercising

17/ Step 2) Virus-laden aerosols mix in the room air
(We are NOT considering close-proximity transmission, since it can't infect a lot of people and lead to a large superspreading event -- Need > 15 min with each person per @CDCgov, just no time in the events analyzed)
(We are NOT considering close-proximity transmission, since it can't infect a lot of people and lead to a large superspreading event -- Need > 15 min with each person per @CDCgov, just no time in the events analyzed)

18/ Step 3) Virus in the air can:
- go outside w/ ventilation (F_out, speed of ventilation varies hugely across locations)
- be filtered in the room (not shown in this drawing)
- lose infectivity (typically ~1 hr)
- deposit to the ground (tens of min to many hours)
- go outside w/ ventilation (F_out, speed of ventilation varies hugely across locations)
- be filtered in the room (not shown in this drawing)
- lose infectivity (typically ~1 hr)
- deposit to the ground (tens of min to many hours)
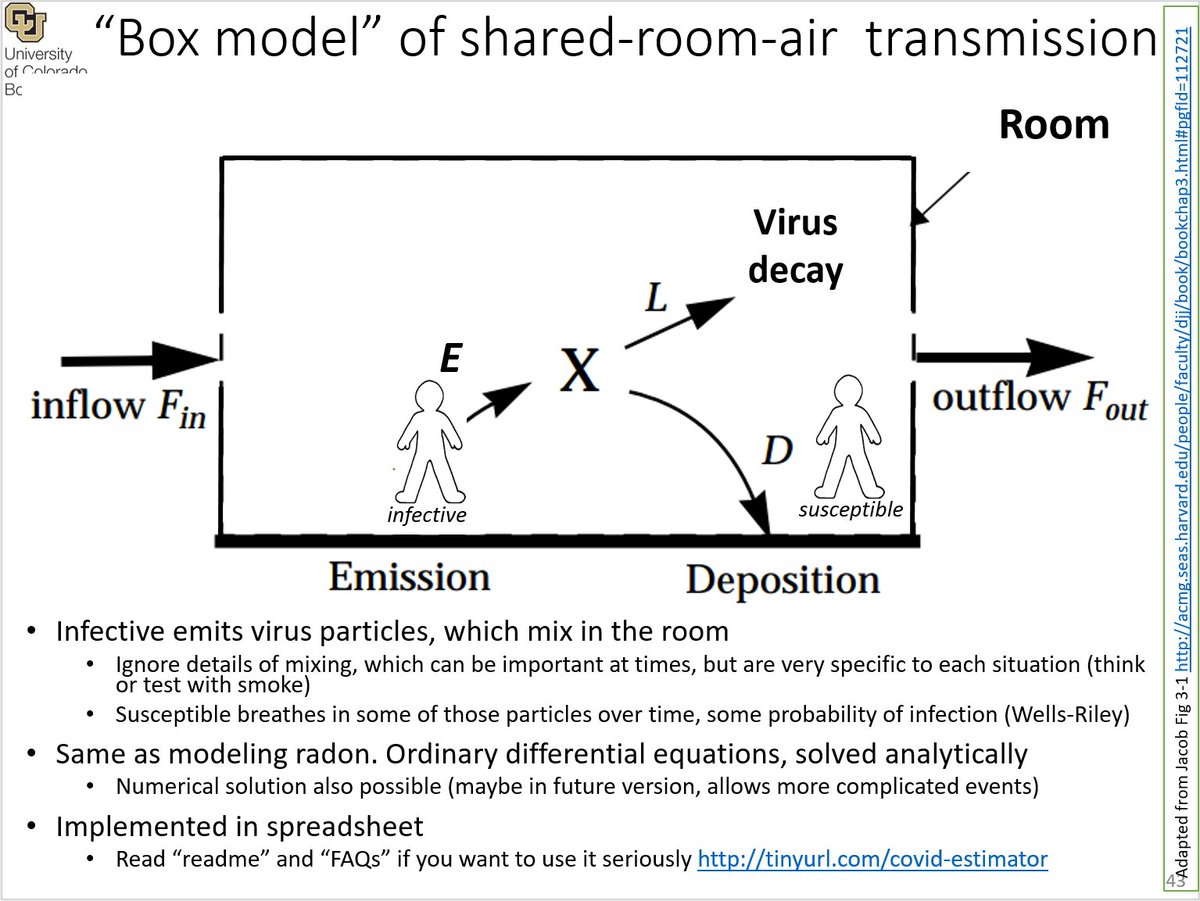
19/ A level of airborne virus is established in the room. Depends on:
- how much virus is being exhaled
- how big the room is
- how quickly is the virus being removed
Analogous to height of water in a sink, depends on flow from faucet, size of sink, and size of drain:
- how much virus is being exhaled
- how big the room is
- how quickly is the virus being removed
Analogous to height of water in a sink, depends on flow from faucet, size of sink, and size of drain:

20/ For those interested in more details and more references, the model is freely-available in spreadsheet format at tinyurl.com/covid-estimator
Useful for those familiar with spreadsheets and quantitative stuff. [Often confusing for those not familiar with those tools]
Useful for those familiar with spreadsheets and quantitative stuff. [Often confusing for those not familiar with those tools]
21/ Multiple very similar models have been published (I'll add a list at the end), they all do the same thing.
But one complexity is that the result depends on many parameters, hard to represent / summarize
But one complexity is that the result depends on many parameters, hard to represent / summarize

22/ This paper makes 2 advances: the first is that we condensed ALL the factors that affect infection into a single number:
- The relative infection risk parameter (Hr)
- The relative infection risk parameter (Hr)

23/ Where:
- r_e: changes in virus emission (e.g. talking, singing)
- r_b: changes in breathing rate ( changes virus inhaled, e.g. exercise)
- f_e and f_i: for how many aerosols escape masks going out of the infected (e) and being inhaled by the susceptible (i) (=1 if no mask)
- r_e: changes in virus emission (e.g. talking, singing)
- r_b: changes in breathing rate ( changes virus inhaled, e.g. exercise)
- f_e and f_i: for how many aerosols escape masks going out of the infected (e) and being inhaled by the susceptible (i) (=1 if no mask)

24/ - D: duration of the event
- V: volume of the room
- lamba_0: ventilation rate
- lambda_cle: air cleaning (e.g. HEPA filter, #corsirosentalbox, UV disinfection)
- See paper for more details
- V: volume of the room
- lamba_0: ventilation rate
- lambda_cle: air cleaning (e.g. HEPA filter, #corsirosentalbox, UV disinfection)
- See paper for more details

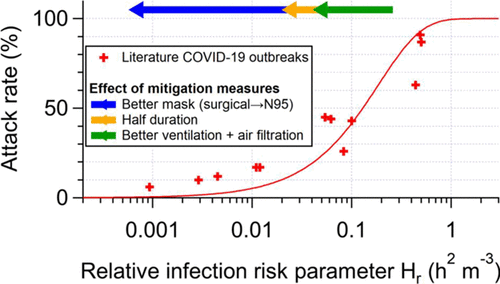
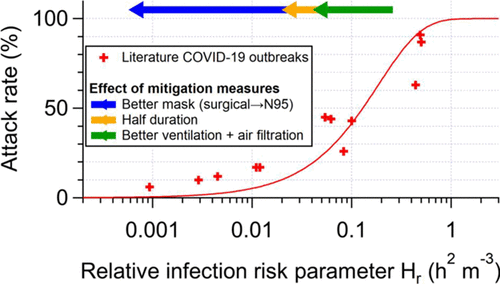
25/ The model predicts that the attack rate (fraction of people present that were infected) should depend on Hr only, for a GIVEN disease.
Does that work? It does for COVID-19!
Red line is model, datapoints are outbreaks
[Ignore mitigation arrows for now]
Does that work? It does for COVID-19!
Red line is model, datapoints are outbreaks
[Ignore mitigation arrows for now]

26/ Agreement is not perfect, but these are outbreaks taken from the literature, with imperfect data about activities etc.
Personally I was astounded when I saw this (@ZheP_AtmChem did a lot of the actual work)
Personally I was astounded when I saw this (@ZheP_AtmChem did a lot of the actual work)

@ZheP_AtmChem 27/ Now, if these superspreading events were caused by e.g. surface transmission, or something else than airborne tr., THERE IS NO REASON why the model would be able to reproduce data
So this confirms that superspreading events are dominated by shared room airborne transmission
So this confirms that superspreading events are dominated by shared room airborne transmission
@ZheP_AtmChem 28/ Why don't we have more events? This is a SORE point.
Bc most investigations done by epidemiologists who do not understand ventilation. They don't report size of the room or ventilation rate.
I've reached out to epi investigators of other outbreaks, most don't even reply.
Bc most investigations done by epidemiologists who do not understand ventilation. They don't report size of the room or ventilation rate.
I've reached out to epi investigators of other outbreaks, most don't even reply.

29/ Now, as Kurt Lewin said, "there is nothing more practical than a good theory". Once the model works, we can use it to:
- analyze other outbreaks
- understand the risk of other events
- quantitatively evaluate mitigation measures
- compare with other diseases
- etc.
- analyze other outbreaks
- understand the risk of other events
- quantitatively evaluate mitigation measures
- compare with other diseases
- etc.

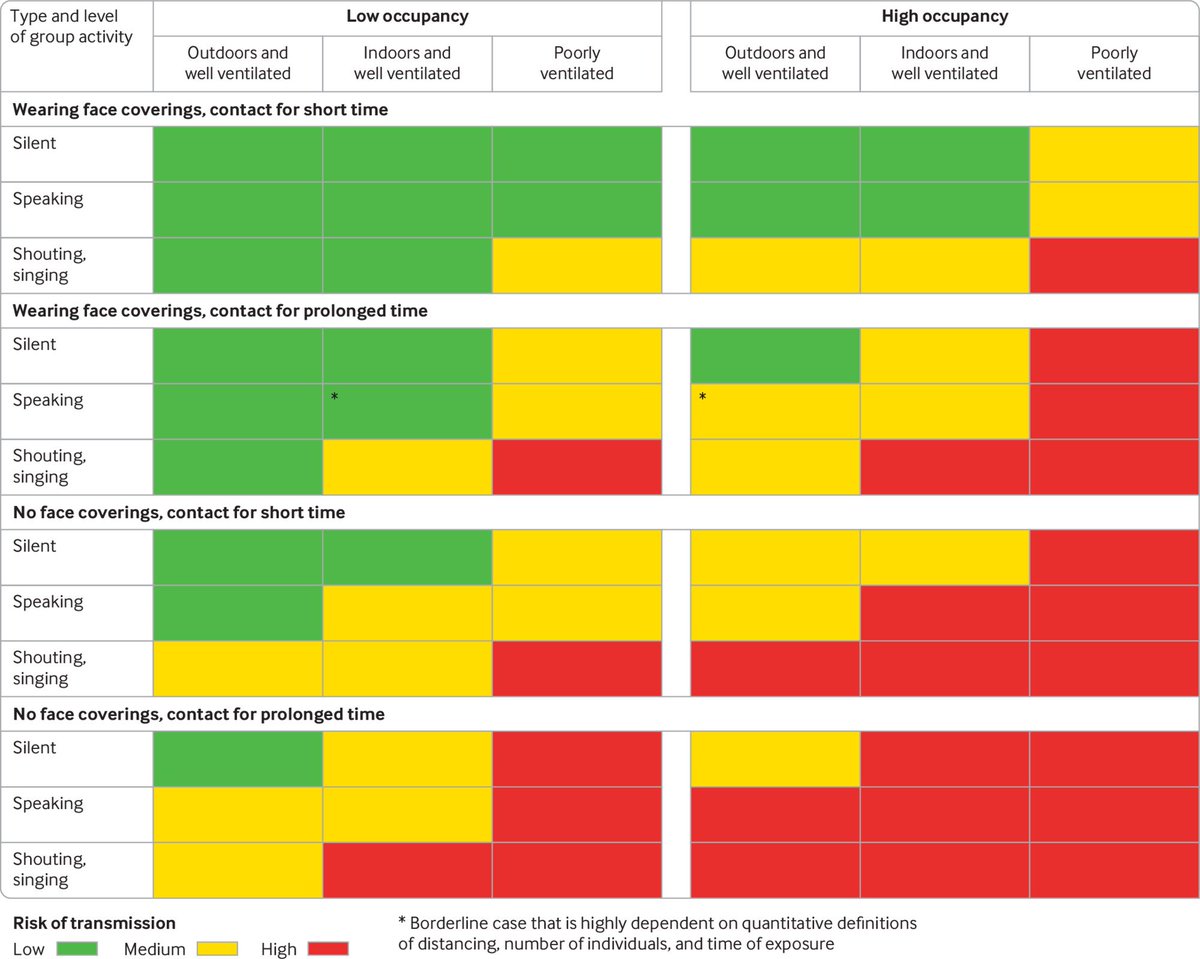
30/ For example, in this table we estimate the risk of transmission of different situations, for the omicron variant with a 1% infection rate (approx. now in UK and US)
[You can tweak it for your situation at tinyurl.com/covid-tables]
[You can tweak it for your situation at tinyurl.com/covid-tables]

31/ The table shows the probability of infection for an individual that participates in an event.
- There may or may NOT be someone infected there:
e.g. if 10 people present and 1% of population infected, then 90% of the time no one is infected, 10% of the time 1 infected
- There may or may NOT be someone infected there:
e.g. if 10 people present and 1% of population infected, then 90% of the time no one is infected, 10% of the time 1 infected

32/ The rest of the factors are the ones that we have discussed already:
- low vs high occupancy (more people, more change someone infected)
- ventilation rate (outdoors vs indoors good vs indoors poor). Note that poor ventilation is the NORM, not the exception, even in USA
- low vs high occupancy (more people, more change someone infected)
- ventilation rate (outdoors vs indoors good vs indoors poor). Note that poor ventilation is the NORM, not the exception, even in USA

33/ - Wearing masks or not (masks remove exhaled virus and prevent inhalation of virus from the air)
- Vocalization: much more virus exhaled if talking, shouting, exercise
- Contact time: more virus accumulates in the air, and occupants breathe more air over longer time
- Vocalization: much more virus exhaled if talking, shouting, exercise
- Contact time: more virus accumulates in the air, and occupants breathe more air over longer time

34/ This table updates a similar, but QUALITATIVE table published by @trishgreenhalgh, Lydia Bourouiba, and colleagues in a paper in @bmj_latest in 2020, that most of you have probably seen.
We collaborated w/ them using model for new QUANTITATIVE table
bmj.com/content/370/bm…
We collaborated w/ them using model for new QUANTITATIVE table
bmj.com/content/370/bm…
35/ Another application of the model is to evaluate the impact of mitigation measures.
I.e. we are told to spend less time, better mask, more ventilation, add HEPA filter. Confusing, one gets tired after doing a few things
How much is enough? How much is risk reduced?
I.e. we are told to spend less time, better mask, more ventilation, add HEPA filter. Confusing, one gets tired after doing a few things
How much is enough? How much is risk reduced?

36/ For example here we start with a high risk situation
By using N95 masks (with attention to fit), halving the duration, and improving ventilation and filtration, we get to a situation where the probability of outbreak is quite low.
Can rank benefit per $$ of each measure
By using N95 masks (with attention to fit), halving the duration, and improving ventilation and filtration, we get to a situation where the probability of outbreak is quite low.
Can rank benefit per $$ of each measure

37/ In the paper we show similar mitigations for a range of locations, that comply with recent ASHRAE (@ashraenews) guidelines.
Which is typically a BEST case scenario. Older places have less ventilation. And compliance only at construction, often things degrade
Which is typically a BEST case scenario. Older places have less ventilation. And compliance only at construction, often things degrade

38/ For example:
- A lot harder to make choir safe: a lot of virus emission by singing, more breathing
- Much easier to make library safe: much lower virus emission since no talking, sedentary, also inhaling less air
Consistent w/ tons of choir outbreaks, none in libraries TMK
- A lot harder to make choir safe: a lot of virus emission by singing, more breathing
- Much easier to make library safe: much lower virus emission since no talking, sedentary, also inhaling less air
Consistent w/ tons of choir outbreaks, none in libraries TMK

39/ Finally, the model allows comparison of different airborne diseases:
- Pulmonary tuberculosis is ONLY airborne
- Measles superspreading is dominantly airborne
- Flu is also airborne (science.org/doi/10.1126/sc…)
- Pulmonary tuberculosis is ONLY airborne
- Measles superspreading is dominantly airborne
- Flu is also airborne (science.org/doi/10.1126/sc…)

40/ COVID-19 has intermediate transmissibility between tuberculosis and measles
Both are airborne. Which is why the old argument (made by e.g. @WHO experts aricjournal.biomedcentral.com/articles/10.11…) "COVID-19 can't be airborne because it is less contagious than measles" never made any sense
Both are airborne. Which is why the old argument (made by e.g. @WHO experts aricjournal.biomedcentral.com/articles/10.11…) "COVID-19 can't be airborne because it is less contagious than measles" never made any sense

41/ We couldn't find many outbreaks in the literature for other diseases that DID have ventilation rate, room volume, and the other parameters we needed.
But relative trend is clear.
[If you know of other outbreaks w/ that info, email me the papers]
But relative trend is clear.
[If you know of other outbreaks w/ that info, email me the papers]

42/ The flu outbreak is the famous case in an airplane that was parked in a tarmac for 3 hours without ventilation, with someone coughing continuously:
academic.oup.com/aje/article-ab…
academic.oup.com/aje/article-ab…
https://twitter.com/jljcolorado/status/1391216930309087234
43/ IMPORTANT: all of the outbreaks included in the paper are from early in the pandemic (it takes time to do the analysis and publish it), so they are all for the original (Wuhan) variant(s) 

44/ We can estimate the impact of the omicron variant, from recent reports of its increased transmissibility. Uncertain, clearly worse, but not like the few historical measles outbreaks yet (subject to update once we have O data):
- Wuhan: central red line
- Omicron: pink line
- Wuhan: central red line
- Omicron: pink line

45/ So I think that's all I wanted to say. Thank you if you made it to here. For more info on the overall airborne transmission thread, you can see this other thread, and papers and threads linked therein:
https://twitter.com/jljcolorado/status/1461356173081276420
46/ If you mostly care about what you can do to protect yourself (hopefully it makes a little more sense now after reading about the model -- since if you made it here, you must be quantitatively minded), see this thread and its links:
https://twitter.com/jljcolorado/status/1461356173081276420
47/ Finally I have to thank many people without whose hard work, input, and feedback this work wouldn't have been possible.
- I started working on this idea when we were working on the paper (led by @ShellyMBoulder) of the Skagit Choir outbreak (onlinelibrary.wiley.com/doi/10.1111/in…)
- I started working on this idea when we were working on the paper (led by @ShellyMBoulder) of the Skagit Choir outbreak (onlinelibrary.wiley.com/doi/10.1111/in…)
48/ I kept working on it, but as they say in my native Spain, I was "slower than the horse of the bad guy" in a Western movie.
I asked @ZheP_AtmChem to work on it w/ me. He did enormous amount of careful work to distill the results.
I couldn't believe my eyes how well it worked
I asked @ZheP_AtmChem to work on it w/ me. He did enormous amount of careful work to distill the results.
I couldn't believe my eyes how well it worked

49/ Many other scientists also participated (and were very patient with me), most from Lidia Morawska's "group of 36" that met with @WHO on 3-Apr-2020, led the "Letter of the 239 scientists" etc.
Including @ShellyMBoulder, @linseymarr, @WBahnfleth, @CathNoakes, @xqcgeo...
Including @ShellyMBoulder, @linseymarr, @WBahnfleth, @CathNoakes, @xqcgeo...
50/ (authors continued)
... @Marcel_Loomans Giorgio Buonanno, Lidia Morawska, Stephanie Dancer, Yuguo Li, Bill Nazaroff, Chandra Sekhar, Raymond Tellier, Julian Tang.
@apinedarojas and @KropffLab joined to help us treat uncertainties more rigorously, but had ton of useful input
... @Marcel_Loomans Giorgio Buonanno, Lidia Morawska, Stephanie Dancer, Yuguo Li, Bill Nazaroff, Chandra Sekhar, Raymond Tellier, Julian Tang.
@apinedarojas and @KropffLab joined to help us treat uncertainties more rigorously, but had ton of useful input
51/ As I said earlier we twisted the arms of @trishgreenhalgh and Lydia Bourouiba to join us to develop a quantitative version of the infection table 

@trishgreenhalgh 52/ If you want to use the estimator at tinyurl.com/covid-estimator, see the readme and FAQ pages.
A video explaining an earlier version of the estimator is here:
A video explaining an earlier version of the estimator is here:
53/ A version of the estimator video in Spanish is at:
There are other related videos in my YouTube channel:
youtube.com/c/JoseLuisJime…
There are other related videos in my YouTube channel:
youtube.com/c/JoseLuisJime…
54/ That's it for now. (I still need to add links for other estimators, but fam. waiting for me to finish to have dinner, patience is thin!)
Again the paper is at
pubs.acs.org/doi/full/10.10…
If you want to ask questions, pls add the tag #Hpaper so that I can find them and reply
Again the paper is at
pubs.acs.org/doi/full/10.10…
If you want to ask questions, pls add the tag #Hpaper so that I can find them and reply
55/ For those of you that use the estimator (Bit.ly/c-est):
- If you use the risk tables only, previous versions are fine (though v. 3.6.2 is easier to understand)
- If you use the spreadsheets, please download the new version (v. 3.6.4), has some important updates
- If you use the risk tables only, previous versions are fine (though v. 3.6.2 is easier to understand)
- If you use the spreadsheets, please download the new version (v. 3.6.4), has some important updates
• • •
Missing some Tweet in this thread? You can try to
force a refresh










