@SabinehazanMD Ok. I start NOW !
handbook to conduct fraudulent repurposed drug covid-19 clinical research
Protocols
1/ Choose wrong drug dosage, too high (HCQ - recovery, solidarity) or too low (IVM - together) depending of the drug safety and efficacy.
1/n
handbook to conduct fraudulent repurposed drug covid-19 clinical research
Protocols
1/ Choose wrong drug dosage, too high (HCQ - recovery, solidarity) or too low (IVM - together) depending of the drug safety and efficacy.
1/n
@SabinehazanMD 2/ Choose wrong treatment duration (IVM - together, etc...),
3/ Choose wrong timing intervention: start late treatment when your trial aim to study an antiviral (HCQ - recovery, solidarity, discovery),
2/n
3/ Choose wrong timing intervention: start late treatment when your trial aim to study an antiviral (HCQ - recovery, solidarity, discovery),
2/n
@SabinehazanMD 4/ Change inclusion criteria regarding delay between symptoms and recruitment (principle: 7 days for all drugs, except for IVM 14 days)
5/ Don't exclude young healthy patients, it'll be hard to see a difference between groups (Lopez-Medina),
3/n
5/ Don't exclude young healthy patients, it'll be hard to see a difference between groups (Lopez-Medina),
3/n
@SabinehazanMD 6/ If it is recommended to take the drug with fat meal, prescribe it on empty stomach (Together), and vice versa
7/ Choose wrong outcome, like 'resolution of all symptoms after 21 days' (Lopez-Medina),
4/n
7/ Choose wrong outcome, like 'resolution of all symptoms after 21 days' (Lopez-Medina),
4/n
@SabinehazanMD 8/ Don't measure viral load if you study early treatment (Lopez-Medina), measure viral load only when you start treatment AFTER the viral phase (HCQ - discovery),
8b/ Argue that not seeing difference in viral load is a bad sign indicating drug doesn't work,
5/n
8b/ Argue that not seeing difference in viral load is a bad sign indicating drug doesn't work,
5/n
@SabinehazanMD 9/ STOP the trials with good protocols if a blatantly fraud (#lancetgate) is published, but continue trials with bad protocols arguing everything is ok (HCQ-recovery),
6/n
6/n
@SabinehazanMD 10/ Argue that your (well-done) study showing non statistically significant 70% death reduction contradicts (poor) meta-analysis showing statistically significant 70% death reduction (Lim),
7/n
7/n
@SabinehazanMD 11/ Don't test multi-therapy (don't forget it's forbidden to save lives using more than one drug), only one single drug.
Meta-analysis
12/ All studies satisfying one of the 1-11/ points is a 'low risk of bias' study.
8/n
Meta-analysis
12/ All studies satisfying one of the 1-11/ points is a 'low risk of bias' study.
8/n
@SabinehazanMD 13/ Don't include multi-therapy trials (don't forget it's forbidden to save lives using more than one drug),
14/ Download data and conduct a rapid meta-analysis before your registration on prospero (Fiolet), make a youtube video and argue it was only pedagogical,
9/n
14/ Download data and conduct a rapid meta-analysis before your registration on prospero (Fiolet), make a youtube video and argue it was only pedagogical,
9/n
@SabinehazanMD 15/ Include observationnal study, until large positive observationnal studies are published, then STOP doing that ! (HCQ)
16/ If results for outpatient and inpatient are different (HCQ), include both in a single meta-analysis to hide positive results,
10/n
16/ If results for outpatient and inpatient are different (HCQ), include both in a single meta-analysis to hide positive results,
10/n
@SabinehazanMD 17/ If results for outpatient and inpatient are positive (IVM), separate both in distinct meta-analysis to hide positive results (Popp)
18/ Include trials that don't match your inclustion criteria if they showed negative results, even if outcome doesn't match (Fiolet),
11/n
18/ Include trials that don't match your inclustion criteria if they showed negative results, even if outcome doesn't match (Fiolet),
11/n
@SabinehazanMD 19/ Evaluate 'low risk of bias' studies published in a high IF journal, and 'high risk of bias' all preprint. Oops high IF are only interested to publish negative paper on repurposed drug 😇,
12/n
12/n
@SabinehazanMD 20/ Trials with positive results are 'high risk of bias', because, you know, we know it's impossible...
21/Choose wrong statistical model, according to what you want to show, don't forget that fixed effect model will produce smaller CI (Shankar-Hari)
13/n
21/Choose wrong statistical model, according to what you want to show, don't forget that fixed effect model will produce smaller CI (Shankar-Hari)
13/n
@SabinehazanMD 21b/ Argue it's ok, because you wrote it in the protocol, nananère!!!
22/ If a trial show positive result, JUST REVERSE BOTH GROUPS (Roman preprint) 😉
23/ Create your own imaginary data on a trial's length of hospital stay (Roman)
14/n
22/ If a trial show positive result, JUST REVERSE BOTH GROUPS (Roman preprint) 😉
23/ Create your own imaginary data on a trial's length of hospital stay (Roman)
14/n
@SabinehazanMD 24/ Write an opposite conclusion to what show the data (Hill),
25/ If someone says you did mistakes and if you fix mistakes it changes the result, argue that you find exactly the same results as other published meta-analysis, thus it's ok not to fix them (Fiolet).
15/15
25/ If someone says you did mistakes and if you fix mistakes it changes the result, argue that you find exactly the same results as other published meta-analysis, thus it's ok not to fix them (Fiolet).
15/15
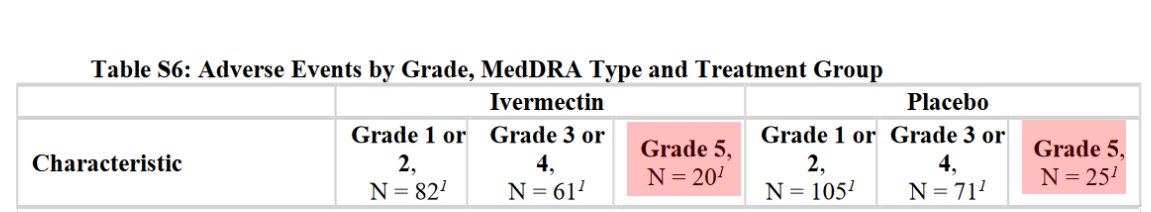
@SabinehazanMD 11b/ Prescribe macrolide to 20% of the control group when your trial is testing azithromycin, a macrolide. Don't speak about it in the study, hide it in the supplementary data (recovery)
@SabinehazanMD People are creative, we have new tactics: 😀
26/ include patients who already recovered in trials whose primary outcome is "symptom duration" (activ6)
26/ include patients who already recovered in trials whose primary outcome is "symptom duration" (activ6)
• • •
Missing some Tweet in this thread? You can try to
force a refresh