This new preprint by Stadler et al. integrated data from 37 randomized controlled trials to ask how the timing and dose of passive antibodies (monoclonal Ab & convalescent plasma) predict protection from SARS-CoV-2 disease. A short 🧵 (1/)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…
Timing: the study found that the earlier the patients were treated with monoclonal antibodies (mAb) or convalescent plasma (CP), the more effective the passive antibodies were in preventing the clinical outcome measured (indicated by right end of line). #TheEarlierTheBetter (2/) 

These data are reminiscent of endogenously induced antibody responses against SARS-CoV-2. In patients with fatal COVID, the onset of antiviral antibodies was significantly delayed compared to those who survived COVID. @carolilucas @sneakyvirus1 (3/)
nature.com/articles/s4159…
nature.com/articles/s4159…
If you break down the data by trial types & mAbs, the pre-exposure injection had the highest efficacy, followed by peri-exposure and later symptomatic stages. mAb use in hospitalized patients provided only limited to no benefits. (4/) 

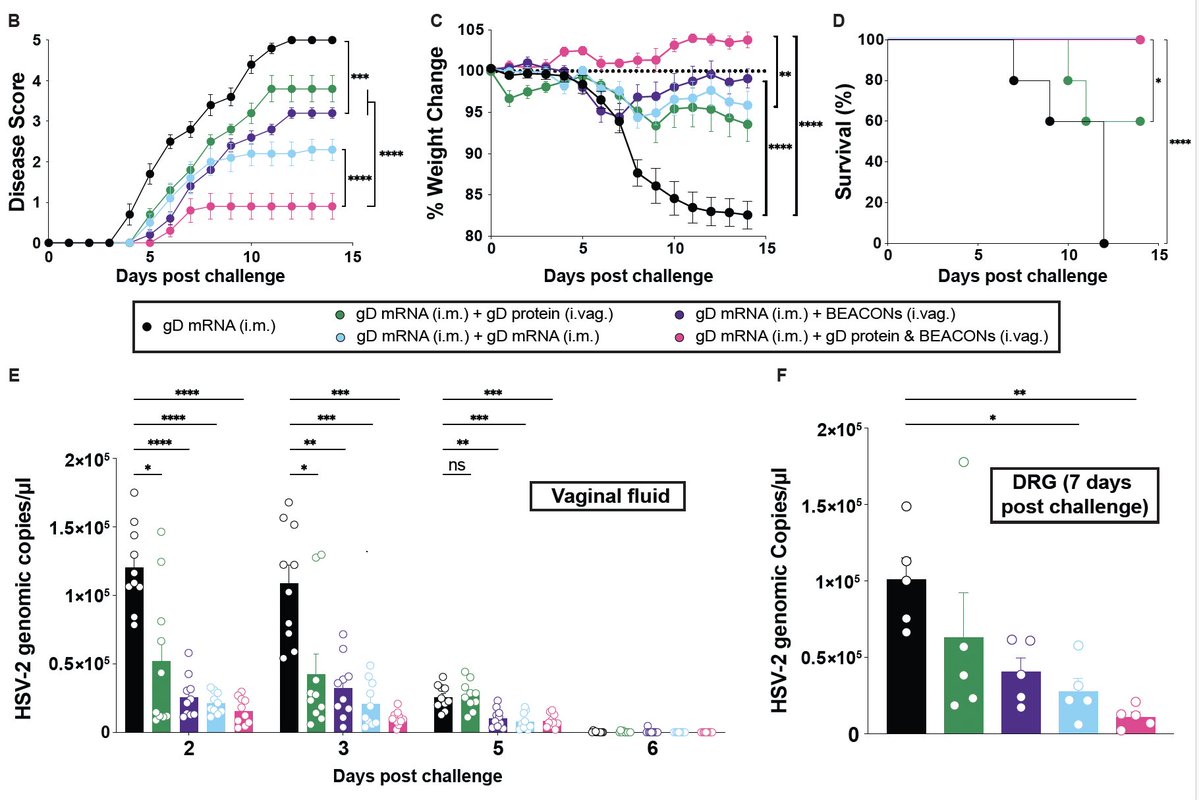
Does the dose of passive antibody matter? This curve shows ‘convalescent equivalent’ of different administered doses of mAb and CP in preventing progression from symptomatic disease to hospitalization. Interesting to note that less is bad but more is not always better. (5/) 
Above data are for ancestral virus. What about Omicron BA.1 and BA.2? Data in this table predict that these mAbs except sotrovimab (green) would fail to neutralize BA.1. Notably, against BA.2, imdevimab at 4000mg dose is predicted to provide 60% protection (yellow).(6/) 

Whether these mAbs provide protection against BA.2 in real world requires clinical studies. However, this type of prediction is very useful in designing clinical trials (timing and dosing) that are most likely to provide benefit to patients. (7/)
To this end, another preprint found that sera of patients receiving Ronapreve (Casirivimab + Imdevimab) and/or Evusheld (Cilgavimab + Tixagevimab) as pre-exposure prophylaxis have some levels of neutralization against BA.2. (8/)
medrxiv.org/content/10.110…
medrxiv.org/content/10.110…

FDA authorized a monoclonal antibody, bebtelovimab, that can work against BA.2 based on lab data. This is great but we need more mAbs that retain neutralizing activities against this and future variants, esp for immunocompromised patients. (9/)
fda.gov/news-events/pr…
fda.gov/news-events/pr…
Paxlovid is great but is contraindicated in patients taking drugs that are highly dependent on CYP3A for clearance. This is because Paxlovid contains ritonavir that inhibits the CYP3A-mediated metabolism of nirmatrelvir (SARS-CoV-2 Mpro inhibitor), but also the following👇🏽(10/) 

There are many other cool data in this preprint. I only highlighted a couple of them but for those interested, please read the original paper for more insights. Congratulations and thanks to the authors of this important study 👏🏼 👏🏼 👏🏼 (end) 

• • •
Missing some Tweet in this thread? You can try to
force a refresh