This is going to be a thread of our #MCBS session starting in a couple of minutes at #ESMO22. Follow along for a LIVE tweet-summary of our session! @myESMO @ChernyNathan @VriesElisabeth 1/
First, @ChernyNathan is giving an overview of cancer drugs approved by FDA in last 5 years. For more, read his excellent paper in @NatRevClinOncol published earlier this year. @DianaNrco 
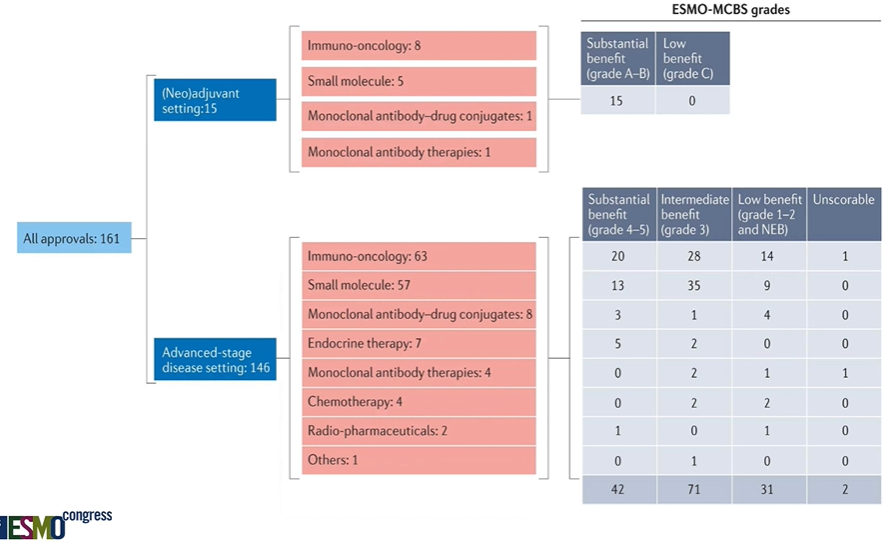
IO drugs made up almost half of these drug approvals. #ESMO22 
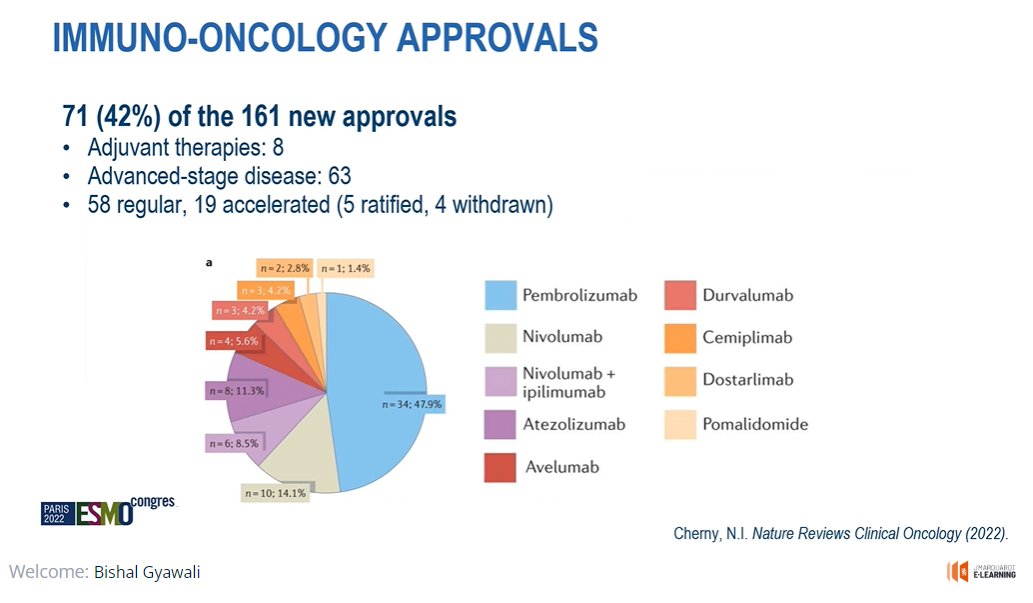
OS benefit a little disappointing because for IO I thought OS data were better than other class of drugs. 

Prof. Cherny asks if we have delivered on the Moonshot Promise. We need more funding for #cancergroundshot to ensure that the innovative drugs actually reach our patients. 

Such a critical point raised by @ChernyNathan . FDA announcements should include critique of trial design, not just repeating the industry announcement. cc @PORTAL_Research 

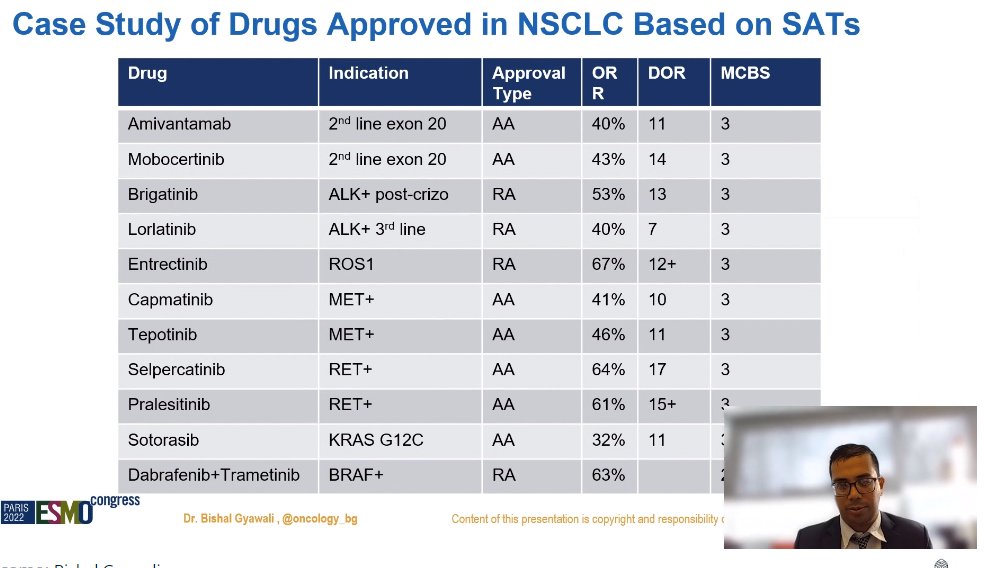
OK, SATs are being used for approval, but they are only conditional approvals, right? Unfortunately, no. 

Why some drugs get regular approval and some get accelerated approval based on similar response rates? I don't know! 

Quoting @sarojniraula et al's study showing RCTs were possible for >80% of the cases where SATs were used for approvals. 

Benefit of IO across tumor types based on MCBS. @LuisCB_MedPharm 

• • •
Missing some Tweet in this thread? You can try to
force a refresh