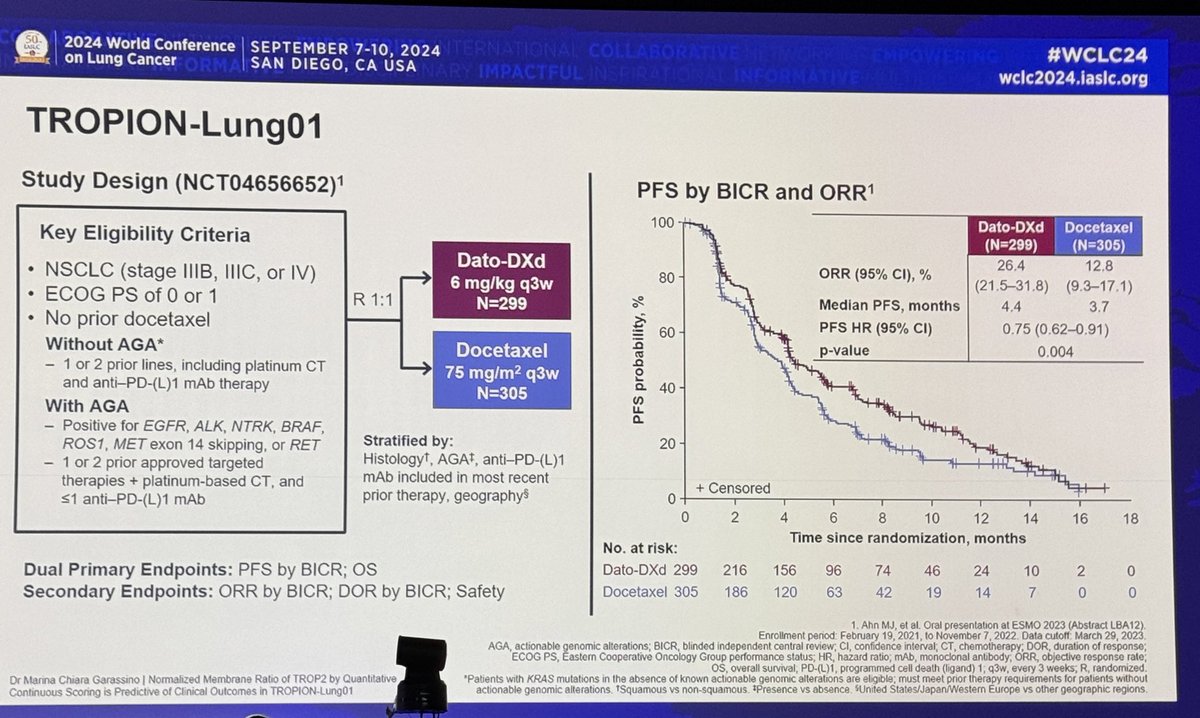
Professor Masahiro Tsuboi presents an important update on ADAURA (adjuvant osimertinib for #EGFR NSCLC) #ESMO22 

The DFS HR of 0.23 in resected stage II/III remains very impressive. But once osimertinib is stopped after 3y, the curves do seem to be coming closer together. DFS at 2y with osi is 90% and at 3y is 84% but drops to 70% at 4y. Are we preventing recurrence or delaying it? #ESMO22 

#ESMO22 in the overall population, similar trends. Off therapy, DFS curves start to approach. Same trends across stages - though I feel more noticeable in stage III (using AJCC 7 or 8). 







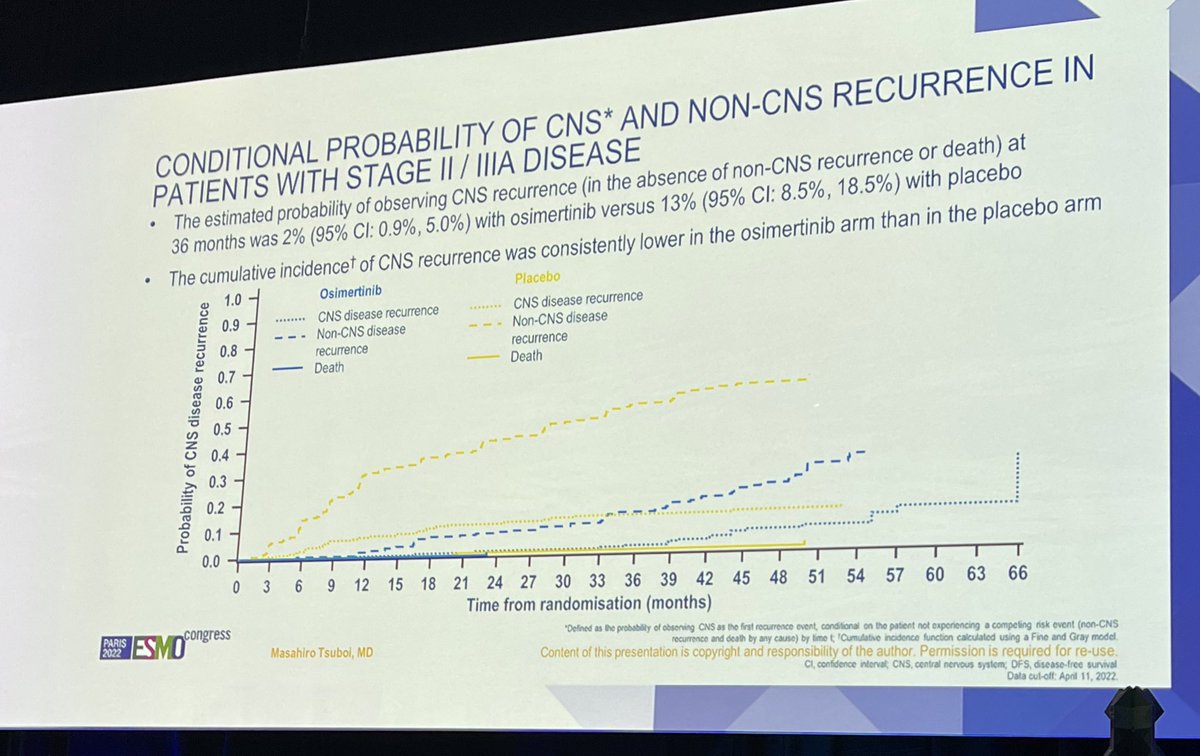
#ESMO22 patterns of recurrence shown here for ADAURA. I would like to see future analyses show differences between on-osimertinib recurrence and off-osimertinib recurrence. Seems to influence CNS in particular. 






#ESMO22 Osimertinib is well tolerated. But the threshold for toxicity can be different in someone who is already possibly cured and in someone asymptomatic at the start of therapy. Only 2/3 in the osimertinib arm completed all three years. 
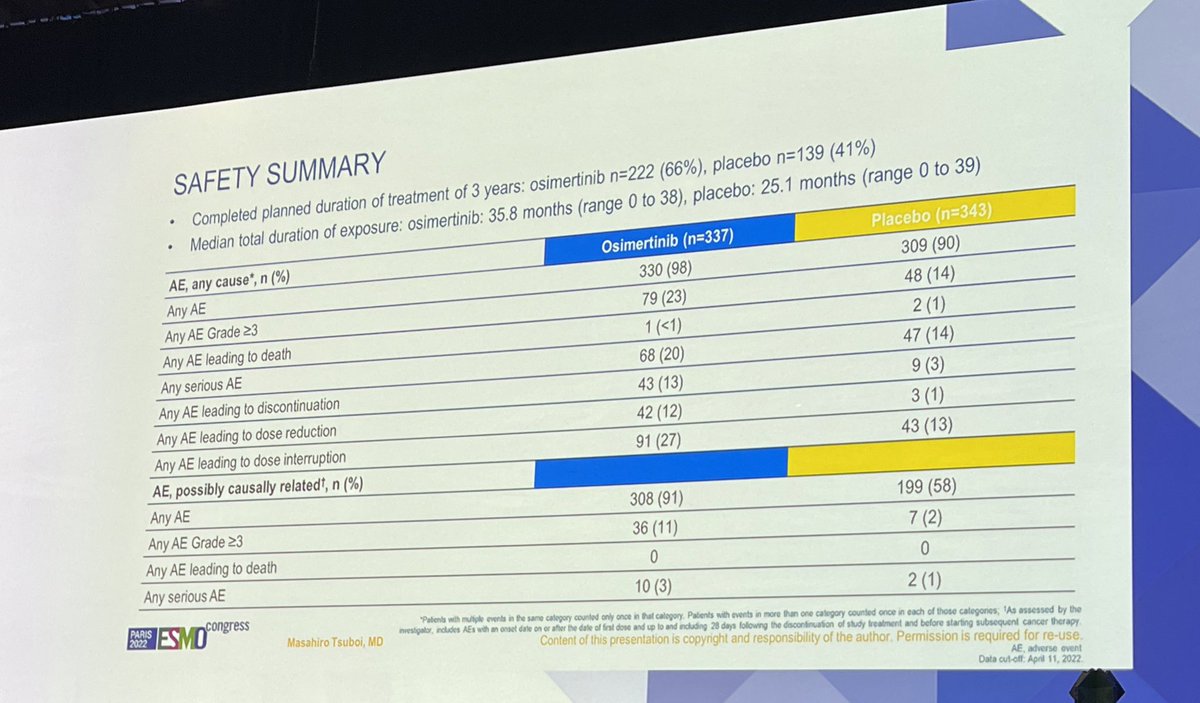
Osimertinib is an effective adjuvant therapy and offers a significant DFS benefit including CNS. But is it curing more people? Will the DFS curves continue to come together? What will the impact on OS be? Stay tuned. #ESMO22 

• • •
Missing some Tweet in this thread? You can try to
force a refresh