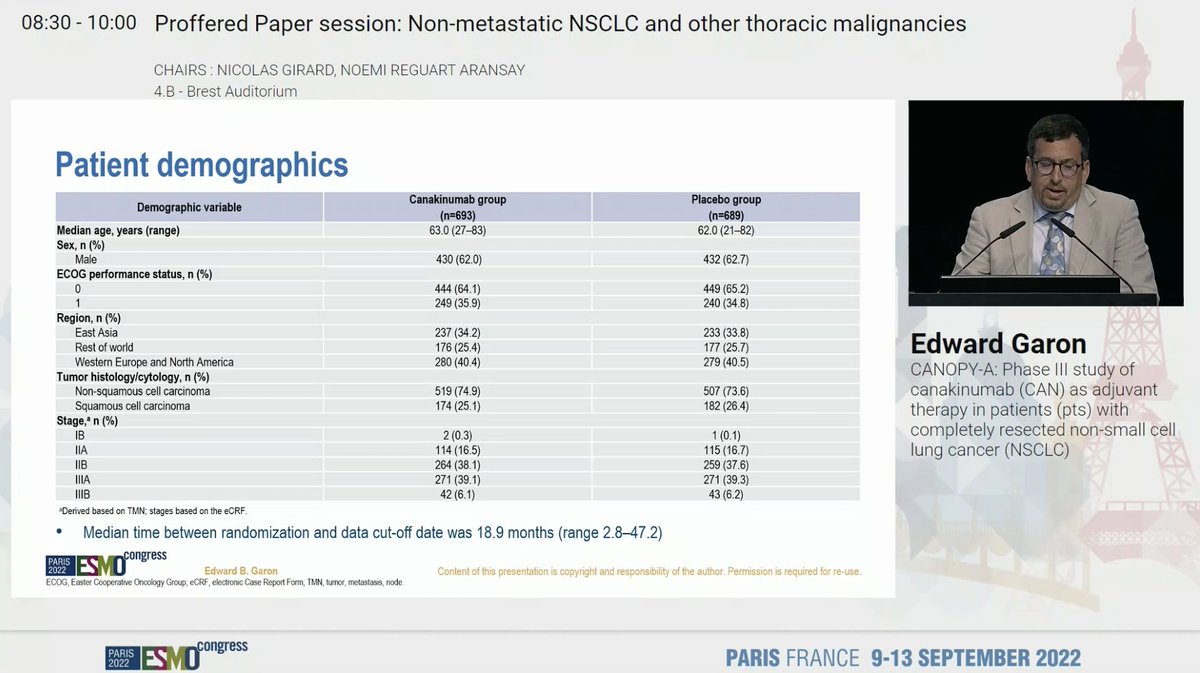
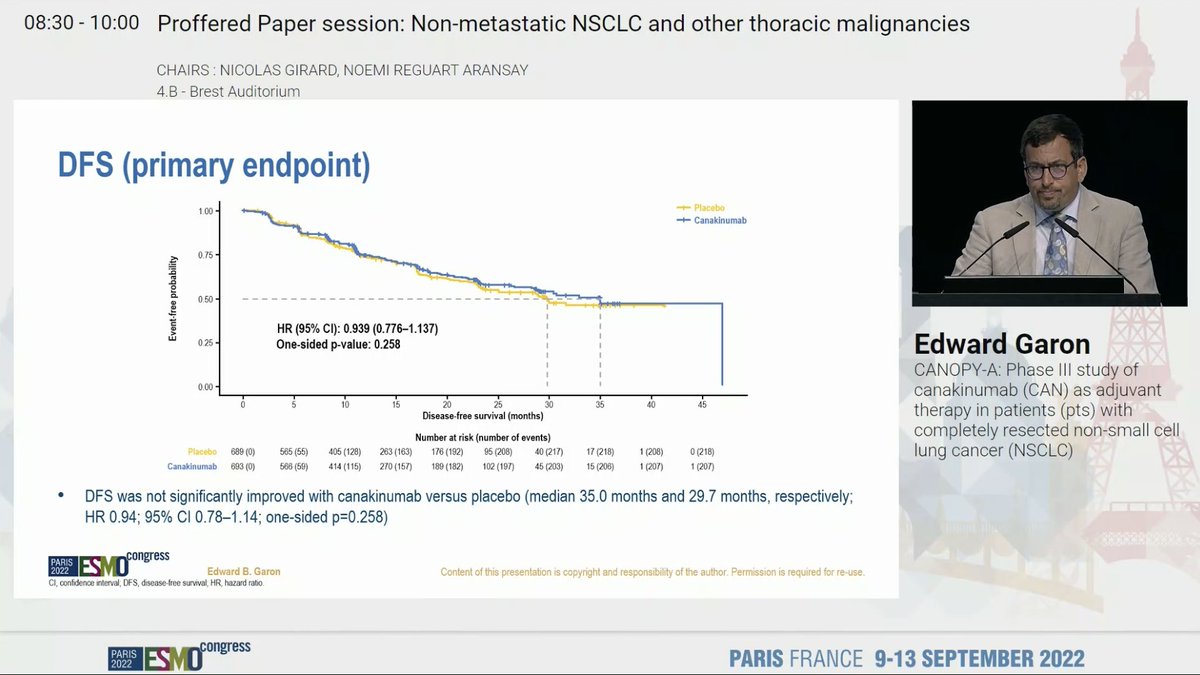
Discussant Dr. @NReguart reminds us of the heterogeneity of stage III NSCLC and our teaching has been to determine resectability before treatment. The INCREASE study starts to challenge that. #ESMO22 

The difference here is radiation and in T3/T4 NSCLC, local control is so critical. Largely unexplored to date. The INCREASE study explores quad-modality therapy and the results were quite impressive. High pCR rates and seen across PDL1 strata, not just in PDL1 high. #ESMO22 







#ESMO22 High G3 TRAE rates but note that use of platinum + etoposide was common and many of these may have been paper toxicities. Any grade pneumonitis only 11% (note radiation dose was usually 50 Gy). 

Good reminder by @NReguart - criteria for resectability is based on expertise of the thoracic surgeon. #ESMO22 

• • •
Missing some Tweet in this thread? You can try to
force a refresh