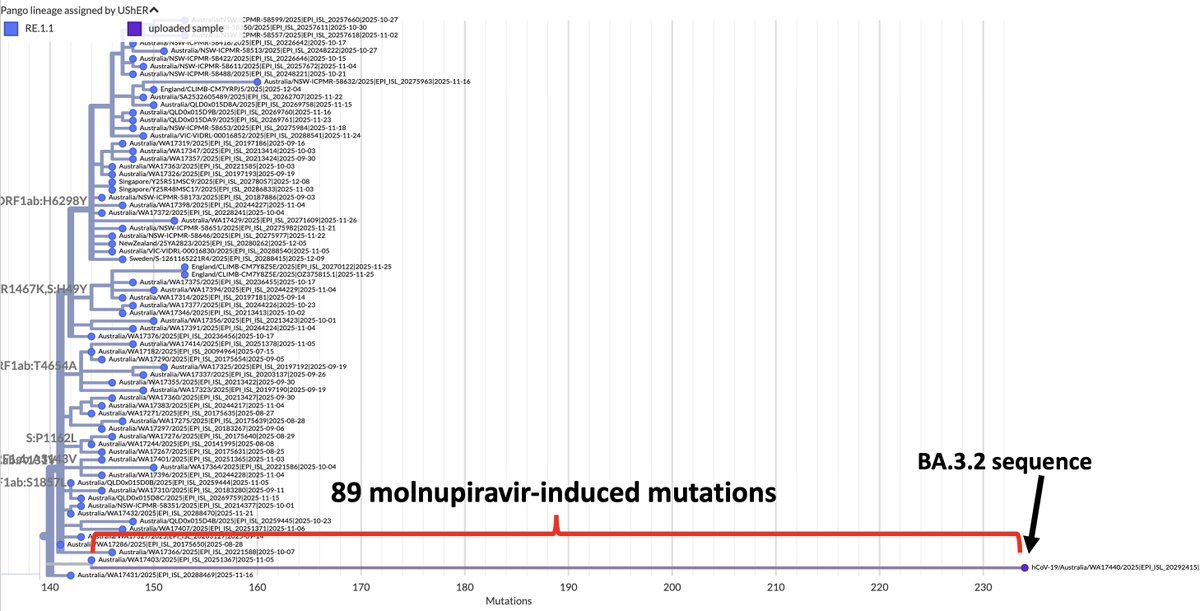
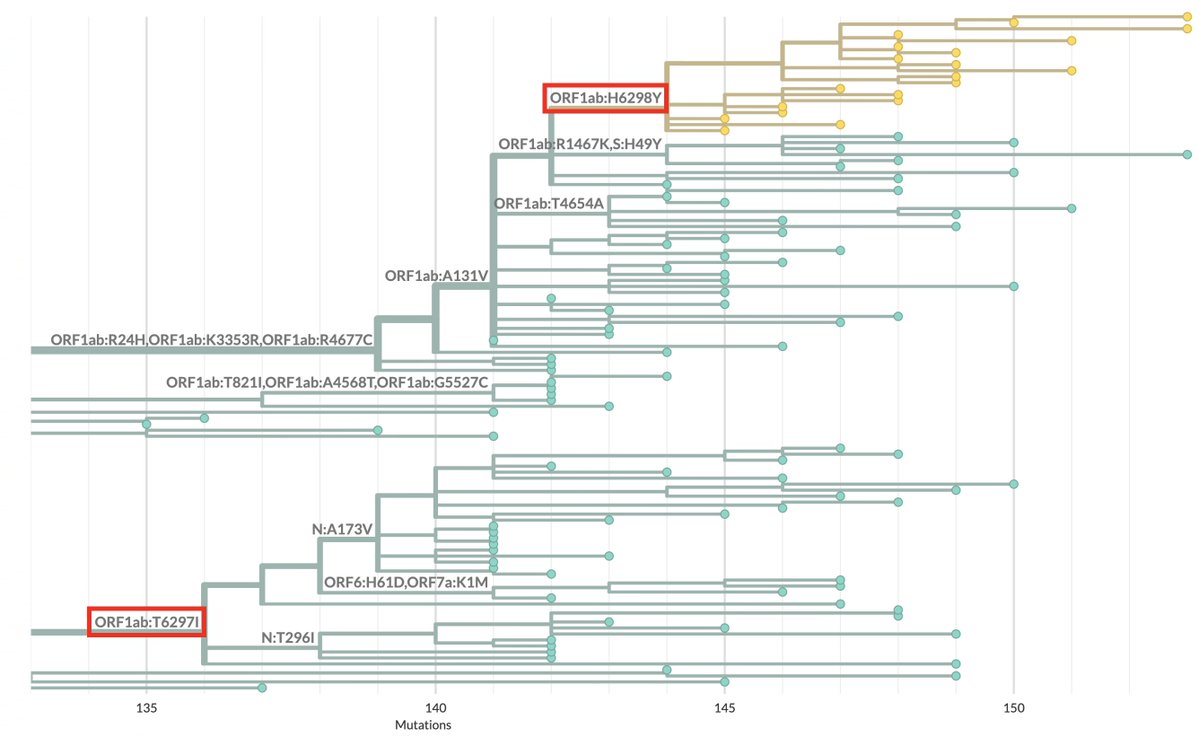
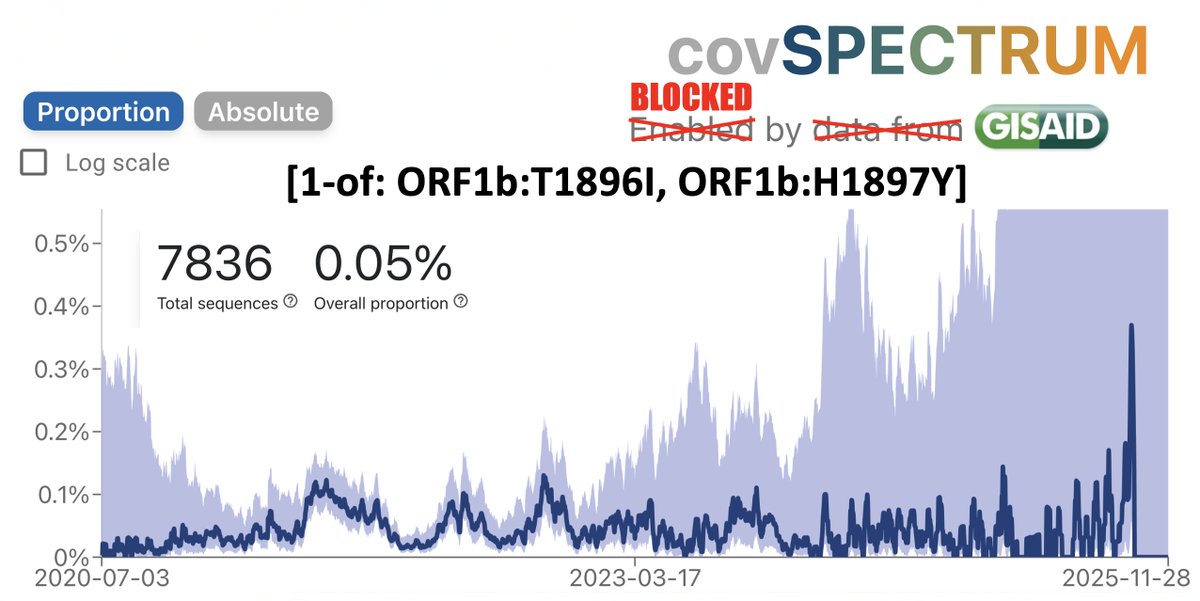
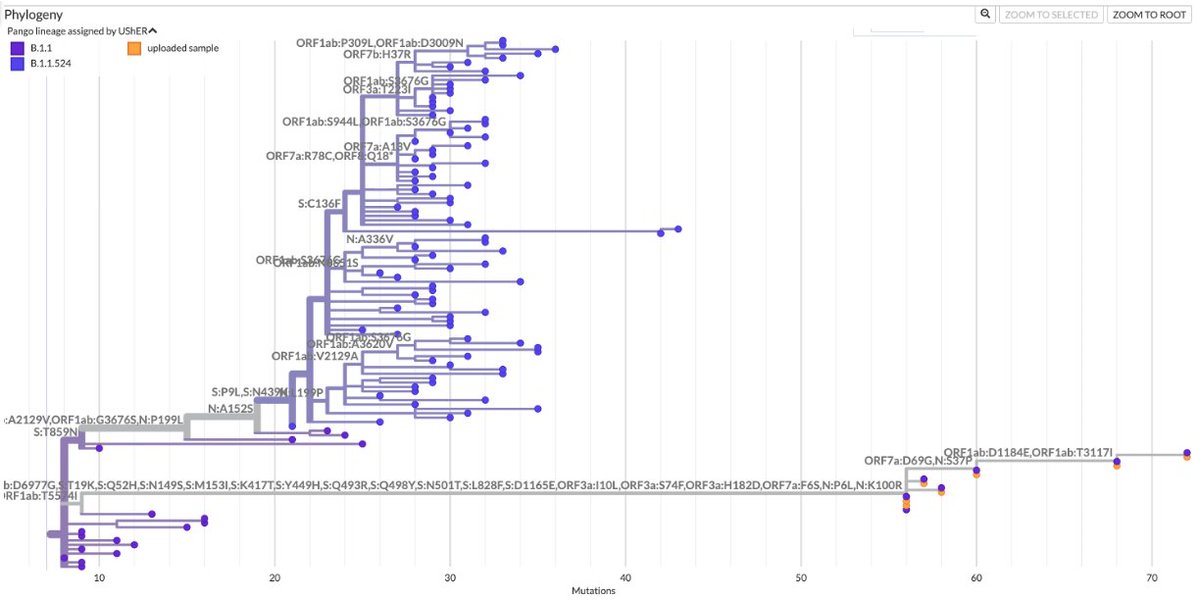
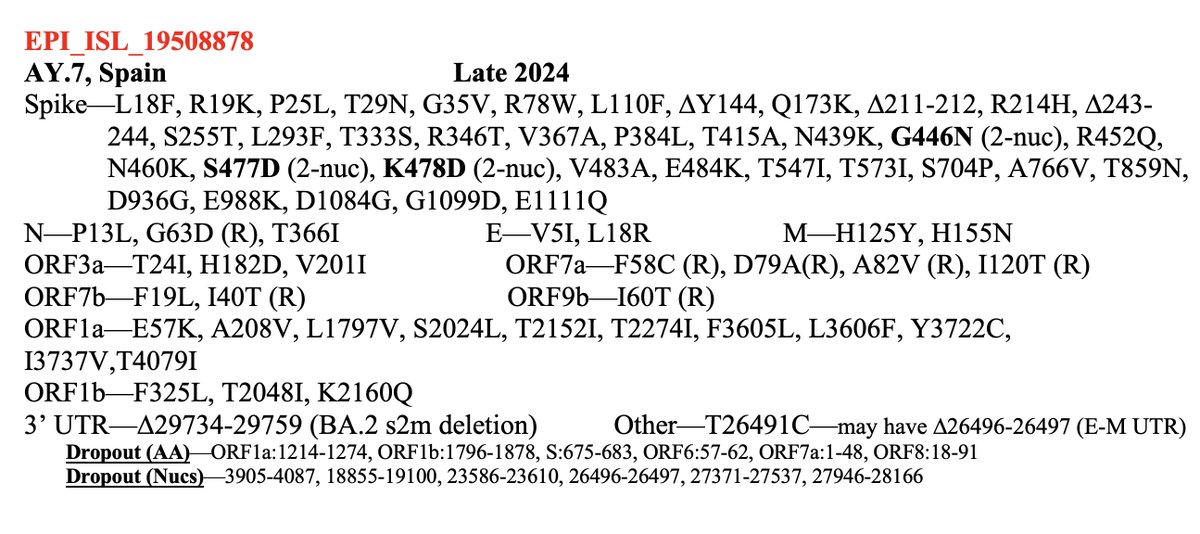
BA.2, driven to near-zero levels by BA.5, still haunt us, spawning monstrous viruses that, after vanishing for months, burst forth, gnarled & hideous, in novel antibody armor. The latest, which I found skulking around India, has 13 spike mutations. 1/28 github.com/cov-lineages/p…
There have been just 4 sequences so far, but they’ve been in Indian cities roughly 800-1300 km apart from each other. Spike mutations consist of three deletions + 10 amino acid substitutions, two of which (K478T & R493Q) are reversions. 2/28 



Most of these mutations are familiar, & known for their antibody-evading properties. The most unique are the K478T reversion & R1091H, an uncommon S2 mutation. But in this thread, I want to discuss the three new deletions, all in the amino-terminal domain (NTD). 3/28 

Most of the info in this thread is from 3 superb studies: 1 by @EnyaQing & co, 1 by @GuptaR_lab, & 1 by @GroveLab. Links below. 4/28 cell.com/cell-reports/f… journals.asm.org/doi/10.1128/mB… embopress.org/doi/full/10.15…
The NTD, at spike-protein amino acid (AA) sites 14-306, is possibly the most variable region in the SARS-CoV-2 genome, but the reasons for NTD mutations are usually less clear than for the receptor-binding domain (RBD, sites 331-528). 5/28 

The RBD binds the ACE2 receptor on human cells & is the primary target of neutralizing antibodies, & most mutations there can be attributed to the effect they have on ACE2 affinity and/or antibody evasion. 6/28
https://twitter.com/trvrb/status/1349774274131738624
The NTD is, to a lesser extent, also targeted by antibodies, primarily at the NTD antigenic supersite. The NTD contains five loops that extend outward, known as N1, N2, N3, N4, & N5. Loops N1, N3, & N5 largely form the NTD supersite. 7/28
https://twitter.com/EricTopol/status/1370460413909893121
At precisely what amino acid sites are these NTD loops located?
• N1 Loop—14-26
• N2 Loop—67-81
• N3 Loop—140-158
• N4 Loop—174-188
• N5 Loop—241-263
embopress.org/doi/full/10.15…
8/28
• N1 Loop—14-26
• N2 Loop—67-81
• N3 Loop—140-158
• N4 Loop—174-188
• N5 Loop—241-263
embopress.org/doi/full/10.15…
8/28

As described below, SARS-CoV-2’s NTD loops are long compared to those of other sarbecoviruses, especially N2, N3, & N5. It is precisely at these loops that we see the vast majority of NTD deletions in SARS-CoV-2. 9/28
https://twitter.com/GroveLab/status/1565375937444724739
NTD deletions, by shortening the length of these projecting loops, can help SARS-CoV-2 evade antibodies, a fact first proven by @mccarthy_kr. He showed this, impressively, before any VOC with deletions had yet emerged. 10/28
science.org/doi/10.1126/sc…
science.org/doi/10.1126/sc…

But there is more to these NTD deletions than just immune evasion. The most common deletion has been ∆69-70—found in Alpha, BA.1, BA.4/5, & others—and it plays little to no role in immune evasion. 11/28 

Variations in NTD-loop length through deletions & insertions turn out to be common in other sarbecoviruses, suggesting their broad utility at facilitating evolutionary change, possibly by compensating for changes caused by mutations elsewhere. 12/28 

For example, @GuptaR_lab found that ∆69-70 usually accompanied RBD mutations (like N439K &Y453F) that strengthen ACE2 binding and/or evade immunity but which also reduce viral infectivity. 13/28 

∆69-70, inserted into pseudoviruses, increased infectivity & when H69/V70 residues were re-inserted back into Alpha, cell entry, S1/S2 spike cleavage (an essential step in entry), & ability to fuse cells (form syncytia) were all greatly impaired. 14/28 



More generally, deletions that shorten NTD loops appear to greatly increase the ability of SARS-CoV-2 to fuse with cell membranes, infect cells, and fuse cells together. 15/28 

SARS1 has much shorter NTD loops than SARS2. @EnyaQing found that replacing the SARS2 NTD with the SARS1 NTD in virus-like particles (VLP) enormously increases its ability to fuse with cell membranes and infect cells. 16/28 

Similar results were found by @GroveLab when replacing SARS-CoV-2’s NTD with that of Pangolin CoV, which has very short NTD loops. 17/28 

By exposing VLP’s to dissolved ACE2 receptors, one can determine the degree to which RBDs are in the exposed “up” position, which is necessary for binding ACE2. The more a VLP is inhibited from fusing w/cell-like particles by dissolved ACE2, the greater the RBD exposure. 18/28 

It turns out that by this measure, SARS-CoV-1 NTD—which, again, has much shorter NTD loops than SARS-CoV-2 & is therefore a good proxy for SARS-CoV-2 viruses bearing many deletions—enhances RBD exposure. 19/28 

So if shorter NTD loops improves cell entry, fusion with cell membranes, the ability to fuse cells together (syncytia formation), and RBD exposure, why don’t all SARS-CoV-2 have large deletions, shortening their NTD loops? 20/28
NTD deletions must exact a cost, & that cost is spike-protein instability. Putting the SARS2+SARS1-NTD VLP’s through a mildly stressful procedure resulted in the loss of the S1 portion of spike, destroying cell entry & fusion capability. 21/28 
So NTD deletions often confer antibody-evading powers and can powerfully increase a virus’s ability to infect cells and fuse cells together, but this comes at the cost of spike instability, leaving it vulnerable to permanent inactivation. 22/28 

What determines whether a given NTD deletion will be deleterious or advantageous? The environment of course, most powerfully, the spike protein background. “The impact of NTD hypervariability depends on the S protein background.” 23/28 
In fact, it was only after the D614G mutation stabilized spike that NTD deletions became possible. The authors of this study—which is impossible to do justice to here—issued an ominous & prescient warning (written in May 2021, before Delta’s properties were known). 24/28
“That a genetic drift around metastable set points can potentially generate hyper-fusogenic CoVs with enhanced cell entry potential is an important consideration in understanding CoV cell entry, transmission, & pathogenicity.” Indeed. 25/28 

Will this particular BA.2 monstrosity, or any of the others, turn out to be hyper-fusogenic, with Delta-like disease severity? My guess is not simply because they retain the Omicron S2, which seems to annihilate all fusogenicity. 26/28
https://twitter.com/LongDesertTrain/status/1565184635256152064
But a reversion to greater fusogenicity, LRT tropism, & Delta-like disease severity seems extremely likely at some point. We would do well to acknowledge this & recognize the necessity of instituting serious NPIs when it happens. 27/28
https://twitter.com/GuptaR_lab/status/1489263774133473286
As usual, I want to emphasize that I’m not an expert in these matters and have no formal credentials. It’s entirely possible I’ve misinterpreted something in this thread, and I welcome corrections and comments from all. 28/28
Finally, thank you to all the hard-working scientists throughout the world sequencing & uploading virus sequences, without which surveillance would be impossible—@r_karyakarte, for example. And thanks to @GISAID for organizing & storing these sequences so they can be monitored.
• • •
Missing some Tweet in this thread? You can try to
force a refresh