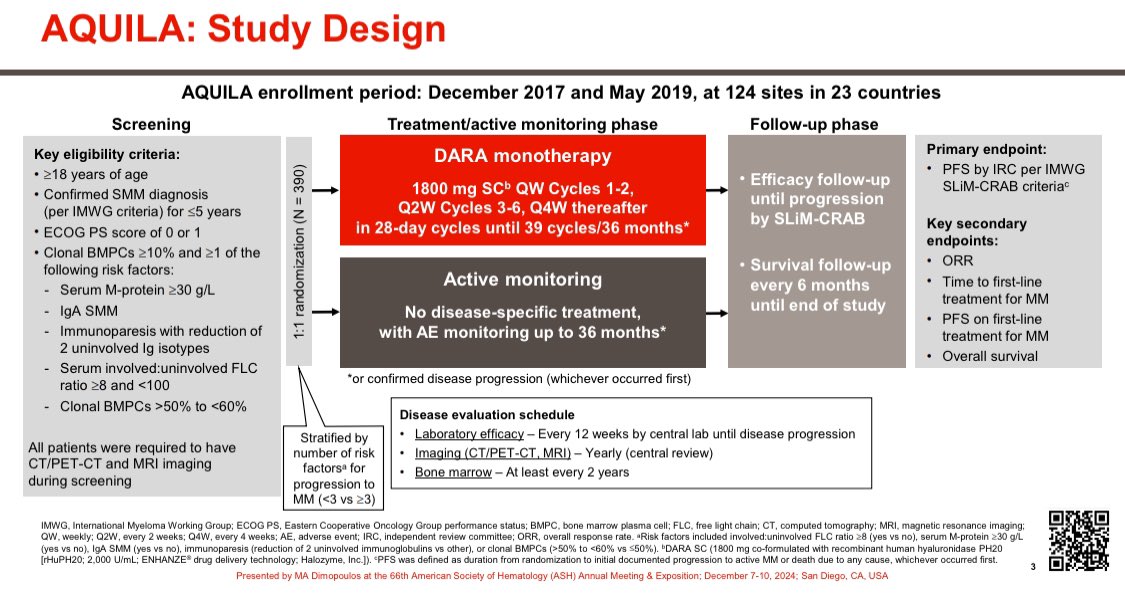
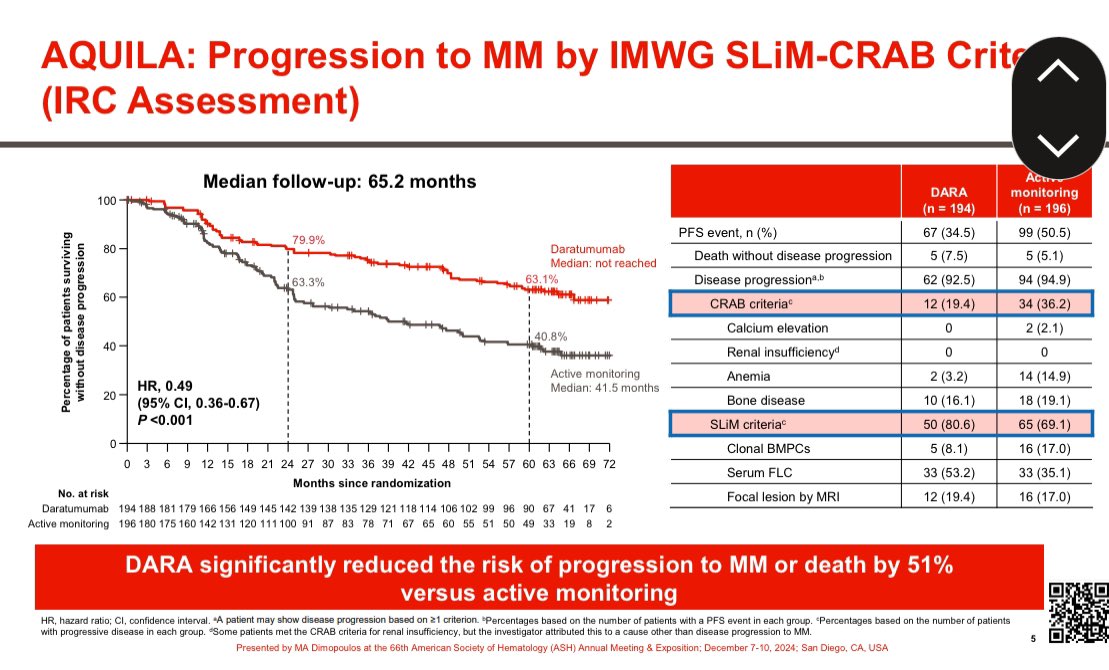
Multiple Myeloma is actually Multiple Myelomas:
At Mayo Clinic, we classify myeloma patients into one of 6 non-overlapping categories based on primary cytogenetic abnormalities that occur at the MGUS stage. #MedTwitter
Needs testing beyond just looking for prognostic markers
1/
At Mayo Clinic, we classify myeloma patients into one of 6 non-overlapping categories based on primary cytogenetic abnormalities that occur at the MGUS stage. #MedTwitter
Needs testing beyond just looking for prognostic markers
1/

Because primary cytogenetic abnormalities occur at the time of origin of MGUS, even if testing was not done at baseline, we can classify myeloma based on a subsequent study.
Thus, a t(4;14) first detected in relapsed myeloma implies presence from the very outset at MGUS stage.
Thus, a t(4;14) first detected in relapsed myeloma implies presence from the very outset at MGUS stage.
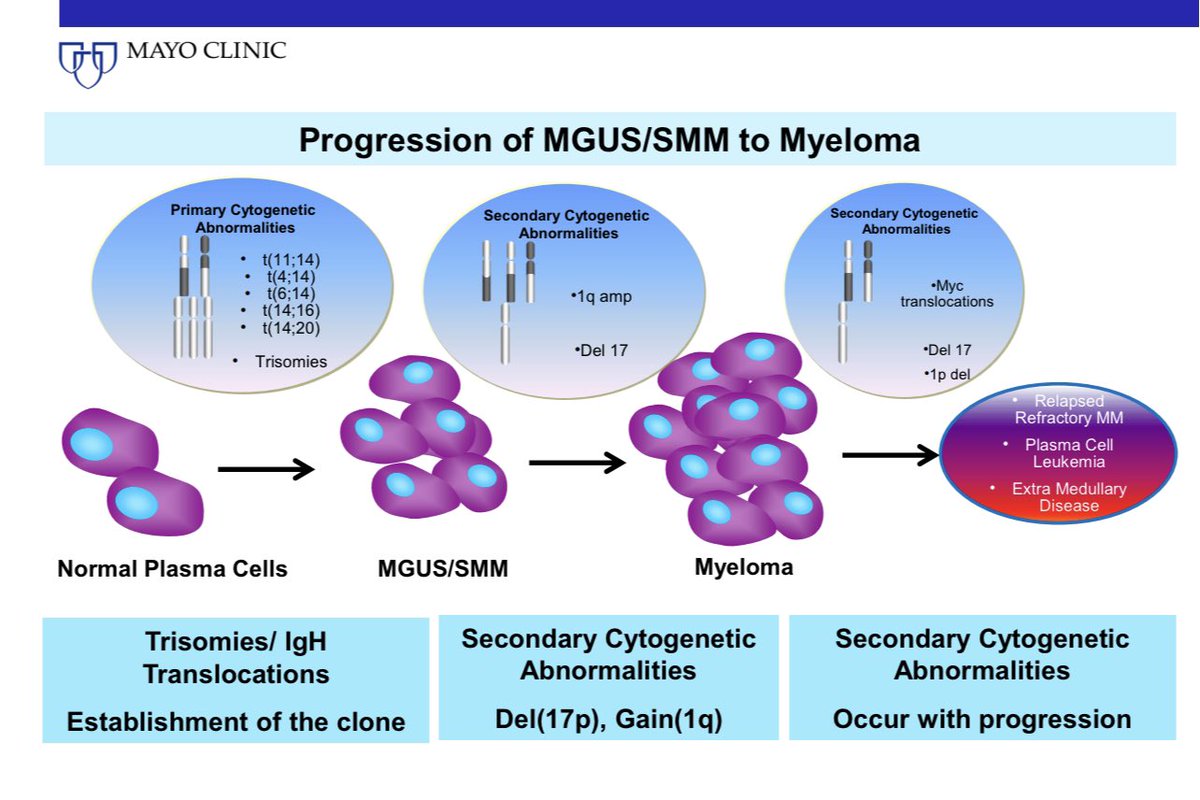
In contrast, secondary cytogenetic abnormalities such as gain 1q, del 17p, del 13, can occur at any time in disease evolution. Further one or more of these secondary abnormalities can occur in each of the 6 main types of myeloma & change their clinical course @NatRevClinOncol
3/
3/

Thus a t4;14 myeloma can develop a del 17p. So can a t11;14 myeloma.
But t4;14 and t11;14 are mutually exclusive.
4/
But t4;14 and t11;14 are mutually exclusive.
4/
The 6 primary types of myeloma differ some in clinical features, response to therapy, & prognosis. t4;14 myeloma is as much different from t11;14 myeloma as CLL is from mantle cell lymphoma.
Right now we consider all of them as one disease, but it’s an over simplification
5/
Right now we consider all of them as one disease, but it’s an over simplification
5/
The future of myeloma will involve identifying optimal ways to manage not just these distinct entities, but go even beyond that. Such as managing t4;14 myeloma with del 17p differently than t4;14 myeloma without del 17p.
6/
6/
As we learn more about the complex genetics of myeloma, in the future we may approach for instance t4;14 with a certain mutation pattern differently that t4;14 with a different mutation signature.
7/
7/
Read more here. @myelomaMD @NatRevClinOncol @MayoMyeloma @MayoCancerCare nature.com/articles/s4157…
8/
8/
The term Multiple Myelomas was first coined by my colleague @Rfonsi1 20 years ago! nature.com/articles/24030…
Note: Presence of a cytogenetic abnormality from a clinical prognosis standpoint has a different implication depending on when it was detected. The effect of an abnormality on MGUS to MM progression is different than impact on MM prognosis @BloodCancerJnl nature.com/articles/bcj20…
The primary cytogenetic abnormalities are disease classifying as well as disease modifying. The secondary abnormalities are disease modifying, and the way and extent to which they modify will depend on the underlying primary cytogenetic type.
There more than 6 primary types of myeloma. But those 6 will comprise almost 90% of myeloma. The rest have IgH translocations involving a partner besides the 5 recurrent ones, or sometimes IgL translocations, etc
Failure to detect one of the 6 types is usually due to inadequate probes to look for them or low number of clonal cells in the marrow sample. Less often due to a primary abnormality besides the 6 main ones.
• • •
Missing some Tweet in this thread? You can try to
force a refresh