Long Thread 👇
Recently, in a piece in the Economic Times, in context of the tragedy in The Gambia, @preddy85 and I discussed the importance of a recall law aimed at withdrawing adulterated or dangerous drugs from the market.
1/n
Recently, in a piece in the Economic Times, in context of the tragedy in The Gambia, @preddy85 and I discussed the importance of a recall law aimed at withdrawing adulterated or dangerous drugs from the market.
1/n

2/n
An essential feature of this is the framing and formulating of a robust drug recall law.
While our government has a system in place for testing drug samples already on the market to assess their quality, there is no system today to mandatorily recall a drug that has
An essential feature of this is the framing and formulating of a robust drug recall law.
While our government has a system in place for testing drug samples already on the market to assess their quality, there is no system today to mandatorily recall a drug that has
3/n
been declared as “Not of Standard Quality” or NSQ by a Govt. Laboratory.
This is a huge issue in a country like India with 37 different regulators who cannot operate outside their respective jurisdictions.
been declared as “Not of Standard Quality” or NSQ by a Govt. Laboratory.
This is a huge issue in a country like India with 37 different regulators who cannot operate outside their respective jurisdictions.
4/n
Consider this:
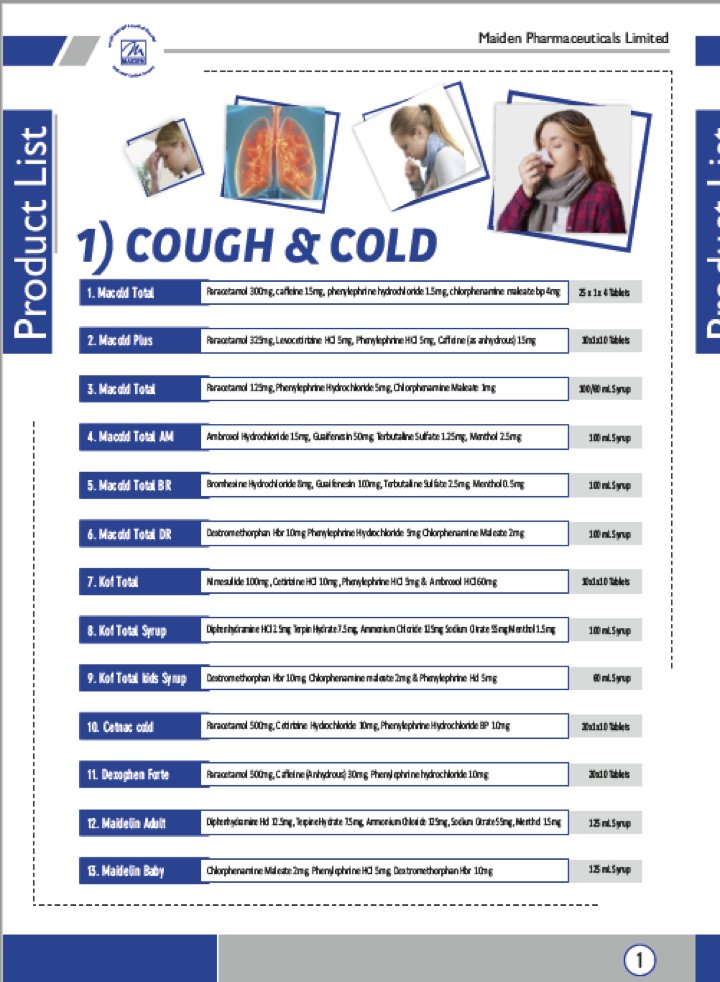
In August 2020, a 2 year old child from Baddi died allegedly due to the consumption of a DEG adulterated cough syrup manufactured by the same company whose cough syrup was responsible for the deaths of the 11 children from Jammu just 8 months earlier.
Consider this:
In August 2020, a 2 year old child from Baddi died allegedly due to the consumption of a DEG adulterated cough syrup manufactured by the same company whose cough syrup was responsible for the deaths of the 11 children from Jammu just 8 months earlier.
5/n
livemint.com/politics/polic…
It is likely that the same batch of adulterated ingredients was used to manufacture different brands of cough syrups which were not effectively recalled the first time when 11 children died earlier.
livemint.com/politics/polic…
It is likely that the same batch of adulterated ingredients was used to manufacture different brands of cough syrups which were not effectively recalled the first time when 11 children died earlier.
6/n
The adulteration and the erring manufacturer were reported to @CDSCO_INDIA_INF, asking for urgent
steps to remove this product from pharmacy shelves.
So why then did a 2 year old have to die 8 months later under similar circumstances?
The adulteration and the erring manufacturer were reported to @CDSCO_INDIA_INF, asking for urgent
steps to remove this product from pharmacy shelves.
So why then did a 2 year old have to die 8 months later under similar circumstances?

7/n
This is how the DCGI responded:
It issued a letter to the drug controllers of States and Union Territories to take necessary action and appropriate steps for stopping the sale and distribution of Cofset AT syrup in the country.
expresspharma.in/dcgi-asks-drug…
This is how the DCGI responded:
It issued a letter to the drug controllers of States and Union Territories to take necessary action and appropriate steps for stopping the sale and distribution of Cofset AT syrup in the country.
expresspharma.in/dcgi-asks-drug…
8/n
Left to themselves, we know that pharma manufacturers will never withdraw their drugs voluntarily because recalls ruins their reputation and creates liability issues for them.
The government knows this very well.
Left to themselves, we know that pharma manufacturers will never withdraw their drugs voluntarily because recalls ruins their reputation and creates liability issues for them.
The government knows this very well.
9/n
Yet, India does not have a drug recall law— and the reason as to why it lacks such a law is a story of epic bureaucratic procrastination and regulatory capture.
This is the 2nd thread on "Regulatory Capture" - the first one from a couple of weeks ago is here 👇
Yet, India does not have a drug recall law— and the reason as to why it lacks such a law is a story of epic bureaucratic procrastination and regulatory capture.
This is the 2nd thread on "Regulatory Capture" - the first one from a couple of weeks ago is here 👇
11/n
Before narrating the story of how we have arrived here, lets first understand why a Recall Law is necessary.
Even well formulated drugs degrade over time. That is why we have a shelf-life and expiration date. Sometimes, batch release does not catch everything may go wrong.
Before narrating the story of how we have arrived here, lets first understand why a Recall Law is necessary.
Even well formulated drugs degrade over time. That is why we have a shelf-life and expiration date. Sometimes, batch release does not catch everything may go wrong.
12/n
In such cases, we need a framework and a process by which we can recall the entire product in the market so that it doesn’t continue to harm people.
Not all recalls are urgent. For example, the @US_FDA guideline says Class I recalls are those that cause immediate harm
In such cases, we need a framework and a process by which we can recall the entire product in the market so that it doesn’t continue to harm people.
Not all recalls are urgent. For example, the @US_FDA guideline says Class I recalls are those that cause immediate harm
13/n
to patients, while Class III recalls are usually for mislabeling.
In the US, the manufacturer is responsible for the recall while the regulator oversees the company’s strategy, assess the adequacy of the recall process.
This approach will not work for us where we have
to patients, while Class III recalls are usually for mislabeling.
In the US, the manufacturer is responsible for the recall while the regulator oversees the company’s strategy, assess the adequacy of the recall process.
This approach will not work for us where we have
14/n
38 regulators, one for each state and the central regulator, the @CDSCO_INDIA_INF
Back to the story: 1976: In a meeting of the Drugs Consultative Committee (DCC), all state drug controllers, the DCGI, and representatives from the @MoHFW_INDIA came together
38 regulators, one for each state and the central regulator, the @CDSCO_INDIA_INF
Back to the story: 1976: In a meeting of the Drugs Consultative Committee (DCC), all state drug controllers, the DCGI, and representatives from the @MoHFW_INDIA came together
15/n
“to review the existing procedure of recall by manufacturers of drugs found deficient”.
cdsco.gov.in/opencms/opencm…
“to review the existing procedure of recall by manufacturers of drugs found deficient”.
cdsco.gov.in/opencms/opencm…

16/n
It was resolved that the Drug controller of the State where the mfg. was located would be responsible for gathering information from all the distributors & then transmitting those details to the other State drug controllers in whose jurisdiction the distributor was located.
It was resolved that the Drug controller of the State where the mfg. was located would be responsible for gathering information from all the distributors & then transmitting those details to the other State drug controllers in whose jurisdiction the distributor was located.

17/n
Unfortunately, this decision was never translated into law via an amendment to either the Drugs and Cosmetics Act 1940 or the Drugs and Cosmetics Rules 1945.
In effect, the issue of recall was left entirely to the individual discretion of state drug controllers.
Unfortunately, this decision was never translated into law via an amendment to either the Drugs and Cosmetics Act 1940 or the Drugs and Cosmetics Rules 1945.
In effect, the issue of recall was left entirely to the individual discretion of state drug controllers.
18/n
1989: 26th meeting of the DCC.
The Secretary of Health and Family Welfare expressed his concern about the recall of drugs found to be hazardous to public health.
He wanted in place a mechanism to ensure complete recalls of such drugs in a span of 72 hours.
1989: 26th meeting of the DCC.
The Secretary of Health and Family Welfare expressed his concern about the recall of drugs found to be hazardous to public health.
He wanted in place a mechanism to ensure complete recalls of such drugs in a span of 72 hours.
19/n
The minutes of the meeting record that a high-powered group under the Chairmanship of the Health Minister of Maharashtra had been constituted by the Central Council of Health to make recommendations on many issues.
We do not know what became of these recommendations.
The minutes of the meeting record that a high-powered group under the Chairmanship of the Health Minister of Maharashtra had been constituted by the Central Council of Health to make recommendations on many issues.
We do not know what became of these recommendations.

21/n
1996: In a meeting of the DTAB (Drugs Technical Advisory Board)
Agenda: To ensure the swift recall of spurious or adulterated drugs, within the shortest time frame.
What followed was a perversion that would perhaps embarrass even the most cynical of bureaucrats.
1996: In a meeting of the DTAB (Drugs Technical Advisory Board)
Agenda: To ensure the swift recall of spurious or adulterated drugs, within the shortest time frame.
What followed was a perversion that would perhaps embarrass even the most cynical of bureaucrats.

22/n
Simply put, it was concluded that it would be too inconvenient for the industry & the bureaucracy if the law mandated mechanisms to ensure the recall of NSQ drugs within a period of 48 hrs, despite it being known that NSQ drugs pose a grave threat to public health.
Simply put, it was concluded that it would be too inconvenient for the industry & the bureaucracy if the law mandated mechanisms to ensure the recall of NSQ drugs within a period of 48 hrs, despite it being known that NSQ drugs pose a grave threat to public health.
23/n
2004: 35th meeting of the DCC.
The matter was sent to a sub-committee for a detailed discussion.
cdsco.gov.in/opencms/opencm…
Given that there is no mention of this sub-committee in future meetings of the DCC, it is safe to presume that the it never submitted its report. 🤷♂️
2004: 35th meeting of the DCC.
The matter was sent to a sub-committee for a detailed discussion.
cdsco.gov.in/opencms/opencm…
Given that there is no mention of this sub-committee in future meetings of the DCC, it is safe to presume that the it never submitted its report. 🤷♂️
24/n
2011: 43rd meeting of the DCC.
When the issue of time sensitive recalls of NSQ drugs came up once again, the DCC came to this conclusion:
2011: 43rd meeting of the DCC.
When the issue of time sensitive recalls of NSQ drugs came up once again, the DCC came to this conclusion:

25/n
And so, yet another sub-committee was set up to look into the very same issue.
Its recommendations were never discussed in future meetings of the DCC, possibly because it never submitted its report.
And so, yet another sub-committee was set up to look into the very same issue.
Its recommendations were never discussed in future meetings of the DCC, possibly because it never submitted its report.
26/n
2012: 59th Report of the Parliamentary Standing Committee on Health and Welfare:
“... There is no effective method of recalling unsold stocks lying in the distribution network. This cannot be allowed to go on…” (contd.) 👇🏼
2012: 59th Report of the Parliamentary Standing Committee on Health and Welfare:
“... There is no effective method of recalling unsold stocks lying in the distribution network. This cannot be allowed to go on…” (contd.) 👇🏼
27/n
“...once a batch of [substandard] drug is…reported to CDSCO, it should issue a press release forthwith & even insert paid ads in the newspapers apart from uploading the information on its website.
Retail chemists should be advised to…return them to local Drugs Inspectors.”
“...once a batch of [substandard] drug is…reported to CDSCO, it should issue a press release forthwith & even insert paid ads in the newspapers apart from uploading the information on its website.
Retail chemists should be advised to…return them to local Drugs Inspectors.”
28/n
The CDSCO then came out with recall guidelines to satisfy the Parliamentary committee: cdsco.gov.in/opencms/export…
Not surprisingly, the Ministry simply omitted mentioning to the Parliament the crucial detail that those recall guidelines lacked the force of law
The CDSCO then came out with recall guidelines to satisfy the Parliamentary committee: cdsco.gov.in/opencms/export…
Not surprisingly, the Ministry simply omitted mentioning to the Parliament the crucial detail that those recall guidelines lacked the force of law
29/n
and were hence not binding on any governmental authority.
The guidelines were very poorly drafted and are quite vague on fixing responsibilities.
For e.g., on the issue of “inter-State” recalls, it merely states:
“For inter-State coordination & communication, the zonal
and were hence not binding on any governmental authority.
The guidelines were very poorly drafted and are quite vague on fixing responsibilities.
For e.g., on the issue of “inter-State” recalls, it merely states:
“For inter-State coordination & communication, the zonal
30/n
offices of CDSCO of their respective jurisdiction are responsible.”
What happens when drug controllers of different states disagree with each other on recalls?
What does it mean to say that the CDSCO is responsible for “interstate co-ordination and communication”
offices of CDSCO of their respective jurisdiction are responsible.”
What happens when drug controllers of different states disagree with each other on recalls?
What does it mean to say that the CDSCO is responsible for “interstate co-ordination and communication”
31/n
when the CDSCO has no administrative authority over state drug controllers?
2016: 50th meeting of the DCC
A two pronged strategy was proposed:
1) To amend the rules and create a legally binding framework for recall.
when the CDSCO has no administrative authority over state drug controllers?
2016: 50th meeting of the DCC
A two pronged strategy was proposed:
1) To amend the rules and create a legally binding framework for recall.
32/n
2) All States to display information about their NSQ drugs on the “SUGAM” portal (an e-governance platform developed for drug controllers).
These were sensible recommendations, yet nothing was translated into the law. 🤷♂️
2) All States to display information about their NSQ drugs on the “SUGAM” portal (an e-governance platform developed for drug controllers).
These were sensible recommendations, yet nothing was translated into the law. 🤷♂️
33/n
And so we come to the last iteration of this "masterly inactivity"
2019: 57th meeting of the DCC: A sub-committee of DCC submitted a report on the issue but still no rules were enacted
And so we come to the last iteration of this "masterly inactivity"
2019: 57th meeting of the DCC: A sub-committee of DCC submitted a report on the issue but still no rules were enacted
34/n
As a result, 46 years after the issue of recalls was first raised at the DCC meeting in 1976, India still lacks a robust law on recalling NSQ drugs.
What does this say about our drug regulator’s attitude towards protecting public health?
As a result, 46 years after the issue of recalls was first raised at the DCC meeting in 1976, India still lacks a robust law on recalling NSQ drugs.
What does this say about our drug regulator’s attitude towards protecting public health?
35/n
This, despite thousands of NSQ drugs being detected every year by government laboratories across India.
Some of them with dangerous impurities ranging from bacterial endotoxins and glass particles, which have a direct adverse consequences to patients.
This, despite thousands of NSQ drugs being detected every year by government laboratories across India.
Some of them with dangerous impurities ranging from bacterial endotoxins and glass particles, which have a direct adverse consequences to patients.
36/n
@preddy85 and I lay this our in great detail in our book, ‘The #TruthPill: The Myth of Drug Regulation in India’.
Read now:
amazon.in/TRUTH-PILL-Myt…
@preddy85 and I lay this our in great detail in our book, ‘The #TruthPill: The Myth of Drug Regulation in India’.
Read now:
amazon.in/TRUTH-PILL-Myt…
• • •
Missing some Tweet in this thread? You can try to
force a refresh