
#PublicHealth activist. Founder @thakurfdn
Author of The Truth Pill: The Myth of Drug Regulation in India.
Available for purchase at: https://t.co/EBjaTZdRsw
How to get URL link on X (Twitter) App

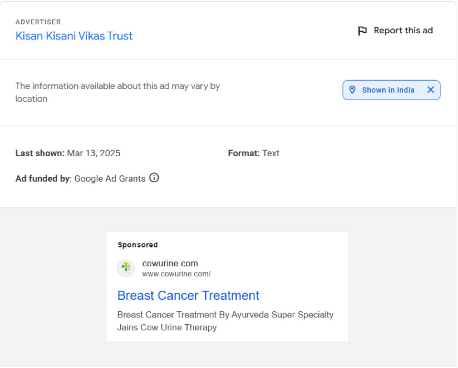

https://x.com/dineshgrao/status/1863948655868408253




 2/n
2/n


 2/n
2/n


 2/n
2/n



 2/2
2/2

 2/n
2/n

 2/n
2/n 

