There is a lot to consider in this first-line study of the #KRAS G12C inhibitor adagrasib plus pembrolizumab from #ESMOImmuno22. Some pleasant surprises in terms of safety. Definitely encouraging but need to see a bit more to be sold on this strategy. 🤔 #LCSM 

We're looking for synergy with the two agents - more than an additive effect. Reason to believe there will be based on preclinical data showing the effect on T-cell infiltration from #KRAS inhibition. Similar to what has been shown with MEK inhibition. #ESMOImmuno22 

The first-line dataset includes the phase Ib KRYSTAL-1 and the phase II KRYSTAL-7. In KRYSTAL-1 (n=7), 4/7 had a response and all were durable (>9m). G3 TRAEs in 4 pts (lipase elevation, LFTs, muscular pain, pneumothorax). #ESMOImmuno22 



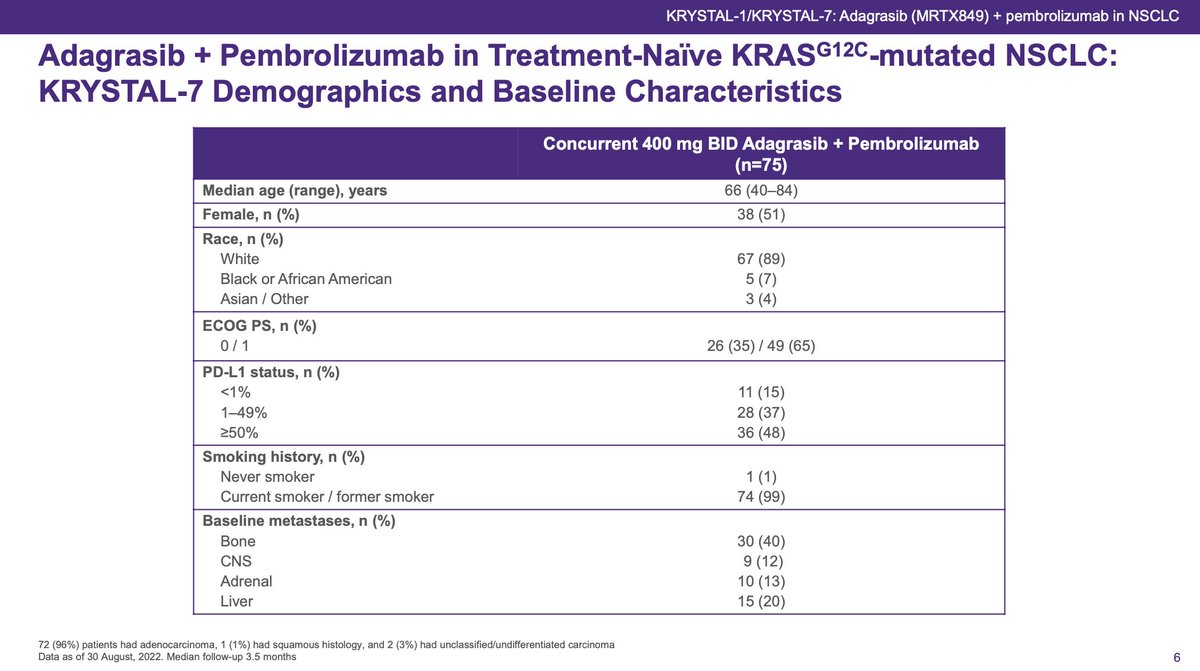
In KRYSTAL-7, 75 pts enrolled with efficacy reported in 53 pts: 14 just enrolled and 8 stopped before the first scan. Catches my eye a little: 1L setting and 19% drop out early including 5 deaths not related to treatment. If death due to PD, that should be included in efficacy. 

#ESMOImmuno22 Half were PDL1 high and nearly all smokers, expected with #KRAS G12C. The safety data are reassuring and better than I would have wagered. Focus on LFTs and <10% G3 with no G4+. TRAEs led to dose reduction in 31% and interruption in 41%. G3+ pneumonitis in 3%. 


KRYSTAL-7: 1L adagrasib + pembrolizumab in #KRAS G12C had RR 49%, DCR 89% with a very impressive waterfall plot. In PDL1 high, RR 59% (13/22) and in PDL1 low, RR 48% (10/21). For PDL1 negative, 3/10 responses. TTR 1.4m and 66% still on therapy. #ESMOImmuno22 





Data shown for pts enrolled for ≥ 6m on KRYSTAL-7. RR in this cohort was 56%. Overall, high response rates and reassuring safety signals. Planning phase III studies of adagrasib + pembrolizumab vs standard IO or chemo-IO. Homerun? Not quite yet, in my opinion. #ESMOImmuno22 



Key is durability, landmark PFS and long-term survival. With IO alone, a subset of pts will be cured (or at least achieve long term survival). Will adding adagrasib increase those cured? If we can induce more CD8+ T-cell infiltration into the tumor and turn cold into hot, maybe!
But if an added agent induces toxicity - will that lead to premature cessation? Will that lead to high dose steroids early in the course? Would that negate the long-term IO effect and leave only the transient targeted therapy benefit? That would be tragic.
I find the combination data encouraging. More appealing in those unlikely to get long-term IO benefit (STK11 etc). For the others - we need to see the long-term impact before we declare success.
"One must wait until the evening to see how splendid the day has been." - Sophocles
"One must wait until the evening to see how splendid the day has been." - Sophocles
• • •
Missing some Tweet in this thread? You can try to
force a refresh