🧵Alberta Health Services (AHS)
Questionable Mask & Respirator Procurement & Selection
💵- Procedural Mask: Orpyx ASTM F2100 L1 or L3
😣- Fit-Tested N95: 3M Cup 8210/8110s
😱- What's the Point KN95: BYD Earloop DG3101
/1



Questionable Mask & Respirator Procurement & Selection
💵- Procedural Mask: Orpyx ASTM F2100 L1 or L3
😣- Fit-Tested N95: 3M Cup 8210/8110s
😱- What's the Point KN95: BYD Earloop DG3101
/1




AHS Questionable Decisions around PPE go far beyond the Infection Prevention and Control (IPC) notorious resistance to #COVIDIsAirborne.
IMO there are Contracting, Procurement & Supply Management (CPSM) and Workplace Health & Safety (WHS) issues as well.
/2
IMO there are Contracting, Procurement & Supply Management (CPSM) and Workplace Health & Safety (WHS) issues as well.
/2
Alberta's Health Care Workers suffered enough from the Vanch mask fiasco in early 2020.
(I am looking forward to what certain regulators or oversight agencies report there, in due course.)
/3
(I am looking forward to what certain regulators or oversight agencies report there, in due course.)
/3
https://twitter.com/ZiadFazel/status/1473937792304443394?s=20&t=d-NfecqwaEFnSjBkypQ84A
In June 2020, Calgary-based Orpyx Medical Technologies, a medical device manufacturer of sensory insoles to help prevent diabetic foot ulcers, and a portfolio company of Western Economic Diversification, announces an interesting partnership.
/4
orpyx.com/orpyx-news/orp…
/4
orpyx.com/orpyx-news/orp…
Capacity for "8 million ASTM-certified, 3-ply procedural face masks a month with room for expansion" is a huge investment to make without a confirmed major customer.
Guess from the announcement who that major customer is
Spoiler Alert: AHS.
/5
orpyx.com/orpyx-news/orp…
Guess from the announcement who that major customer is
Spoiler Alert: AHS.
/5
orpyx.com/orpyx-news/orp…

The urgency around GoA's current pursuit of 5m bottles of children's pain & fever no matter what the business case reminds me of Orpyx contract that was portrayed as "Bits & Pieces" help, but from its early days, was a massive untendered (?) subsidy.
/6
/6
https://twitter.com/ZiadFazel/status/1605797721683759105?s=20&t=d-NfecqwaEFnSjBkypQ84A
Unclear to me why in Summer/Fall of 2020, if GoA wanted to establish domestic manufacturing of PPE, why it invested $60m to be an also-ran in commodity manufacturing of procedural masks.
The need was, and remains, for Made-in-Canada respirators.
/7
kitchener.citynews.ca/local-news/cer…
The need was, and remains, for Made-in-Canada respirators.
/7
kitchener.citynews.ca/local-news/cer…
So let's turn our September 2020 eyes from Procedural Masks
where AHS dropped $60m into a glut of national capacity
to N95 equivalent Respirators
where incumbent 3M could not meet soaring demand from 2nd/3rd/4th Wave.
Bizarre AHS decisions again.
/8
where AHS dropped $60m into a glut of national capacity
to N95 equivalent Respirators
where incumbent 3M could not meet soaring demand from 2nd/3rd/4th Wave.
Bizarre AHS decisions again.
/8
In the above 24 Sep 2020 video, AHS Executive Director of Workplace Health and Safety advises that both 3M N95 at the core of AHS fit-tested respiratory protection were no longer available:
• Aura 3-fold boat design
• VFlex easy-breathing duckbill
/9
multimedia.3m.com/mws/media/1387…
• Aura 3-fold boat design
• VFlex easy-breathing duckbill
/9
multimedia.3m.com/mws/media/1387…
Exec Dir of WHS explains they are filling in temporarily with 3M 8210/8110s cup-style industrial N95..
but instead of diversifying to a Canadian-made replacement, they somehow made Shanghai Dasheng DTC3Z their number one choice, and would be actively fit-testing to that.
/10



but instead of diversifying to a Canadian-made replacement, they somehow made Shanghai Dasheng DTC3Z their number one choice, and would be actively fit-testing to that.
/10




So, AHS feels locally-made flat masks are so important they spend $60m to get $24m of commodity product made in Calgary.
But for even more important respirators, instead of going to Canadian manufacturers innovating with Health Canada, AHS goes to China for 3M Aura clones.
/11
But for even more important respirators, instead of going to Canadian manufacturers innovating with Health Canada, AHS goes to China for 3M Aura clones.
/11
Yes, dear readers, you'll be GOBSMACKED to learn that within a year of AHS commitment to Shanghai Dasheng as #1 respirator for fit testing..
..both US FDA & Health Canada revoked their interim approval of every single Shanghai Dasheng respirator.
/12
fda.gov/medical-device…



..both US FDA & Health Canada revoked their interim approval of every single Shanghai Dasheng respirator.
/12
fda.gov/medical-device…




@TheBreakdownAB @CAPPEM2 @BarryHunt008 @projectn95 @dewigmore @CAAerosolCltn @Protect_BC @PPEtoheros @demandsbetter @Adam_Toy Shared Health Manitoba had fit-tested staff to this Dasheng DTC3Z too. They worked with Health Canada and Workplace Health & Safety Manitoba to:
• independently test inventory
• work out a transition
• communicate with workers
Did AHS do this?
/13
sharedhealthmb.ca/files/covid-19…
• independently test inventory
• work out a transition
• communicate with workers
Did AHS do this?
/13
sharedhealthmb.ca/files/covid-19…
@TheBreakdownAB @CAPPEM2 @BarryHunt008 @projectn95 @dewigmore @CAAerosolCltn @Protect_BC @PPEtoheros @demandsbetter @Adam_Toy In August 2021, Dasheng began a "Latest News" section to try to control and reverse the damage from NIOSH revocation:
• 20 Aug - we were blindsided
• 8 Sep - we should get our certification back within 30 days
• 13 Sep - forget NIOSH, EU Better
/14
dashengmask.com/LatestNews/id/…
• 20 Aug - we were blindsided
• 8 Sep - we should get our certification back within 30 days
• 13 Sep - forget NIOSH, EU Better
/14
dashengmask.com/LatestNews/id/…
By Oct 2021, it should be clear to AHS:
• Dasheng abandoned Canada/US N95 market
• Delta/4th Wave filling up ICUs and burning out HCW
• 3M cup-style N95 with no exit valve (temporary fallback until Dasheng) are hurting HCW over long shifts, eg.
/15
refinery29.com/en-ca/2020/04/…
• Dasheng abandoned Canada/US N95 market
• Delta/4th Wave filling up ICUs and burning out HCW
• 3M cup-style N95 with no exit valve (temporary fallback until Dasheng) are hurting HCW over long shifts, eg.
/15
refinery29.com/en-ca/2020/04/…
So a rational, ethical employer of AHS's size & complexity would realize it urgently needs millions of respirators that are:
• Health Canada approved
• meets Alberta OH&S Code
• comfortable for extended use by HCW
• NOT another Vanch fiasco
/16
albertahealthservices.ca/about/about.as…
• Health Canada approved
• meets Alberta OH&S Code
• comfortable for extended use by HCW
• NOT another Vanch fiasco
/16
albertahealthservices.ca/about/about.as…
Rational, ethical things AHS could have done in Fall 2021:
• Go back to 3M Aura & VFlex ramped up manufacturing to meet HCW demand (they had)
• See the progress of Canada's industry/government collaboration, especially @CAPPEM2 & @GovCanHealth
/17
newswire.ca/news-releases/…
• Go back to 3M Aura & VFlex ramped up manufacturing to meet HCW demand (they had)
• See the progress of Canada's industry/government collaboration, especially @CAPPEM2 & @GovCanHealth
/17
newswire.ca/news-releases/…
@CAPPEM2 @GovCanHealth In Aug 2020, govs of Canada & Ontario each kicked in $23.33m to expand 3M's N95 production in Brockville, ON to 100m/year, with each gov committed to 25m/yr for 5 years.
pm.gc.ca/en/news/news-r…
By April 2021, 3M had produced the first 1 million.
canada.ca/en/innovation-…
/18
pm.gc.ca/en/news/news-r…
By April 2021, 3M had produced the first 1 million.
canada.ca/en/innovation-…
/18
In Fall 2020, Ontario and the feds invested $46.67m for 50m world-class N95/yr from 3M.
Just under a dollar each.
Leaving 50m/yr of capacity.
Did GoA go for it? Not Trudeau, but Kenney & Ford are friends, right?
Nope. GoA dropped $60m on Orpyx flat masks at $1.50 each.
/19
Just under a dollar each.
Leaving 50m/yr of capacity.
Did GoA go for it? Not Trudeau, but Kenney & Ford are friends, right?
Nope. GoA dropped $60m on Orpyx flat masks at $1.50 each.
/19
When I say GoA got $24m of masks for $60m, I am comparing to AHS landed cost for Primed PG4-196612 procedural masks in Summer 2020, when PPE was at a premium, and AHS was desperate, buying volume well above contract price.
Still about $0.60 each.
/20
albertahealthservices.ca/assets/info/pp…



Still about $0.60 each.
/20
albertahealthservices.ca/assets/info/pp…




In summer 2020, it became obvious that Canada would be nearly self-sufficient in PPE manufacturing, especially in manufacturing 3-ply polypropylene flat masks, which is fairly straightforward for the disciplined manufacturers we have across Canada.
/21
cbc.ca/news/politics/…
/21
cbc.ca/news/politics/…
I defer to @BarryHunt008 @CAPPEM2 if they wish to comment, but I understand a contract for 40m Canadian-made ASTM F2100 L1 flat masks over 2 years starting Fall 2020 would be a small fraction of $1.50 each.
If only they had a chance to bid...
/22
cbc.ca/news/canada/ot…
If only they had a chance to bid...
/22
cbc.ca/news/canada/ot…
Let's check our roadmap. I've covered procedural mask and N95.
Not yet KN95 😱
By Fall 2021, GoA & AHS *should have* learned from Vanch, Orpyx & Shanghai Dasheng fiascos.
The urgent need was for fit-tested respirators to replace the 3M 8210 & 8110s cup-style N95.
/23
Not yet KN95 😱
By Fall 2021, GoA & AHS *should have* learned from Vanch, Orpyx & Shanghai Dasheng fiascos.
The urgent need was for fit-tested respirators to replace the 3M 8210 & 8110s cup-style N95.
/23

Don't get me wrong. 3M 8210 & 8110s cup-style N95 (which AHS intended as a temporary stopgap to get them to their preferred supplier Shanghai Dasheng) are not BAD respirators.
I agree with most of what @larmbrust says in his review.
But....
/24
armbrustusa.com/blogs/mask-rev…
I agree with most of what @larmbrust says in his review.
But....
/24
armbrustusa.com/blogs/mask-rev…
@larmbrust But for many reasons, those N95 are not suited to HCW.
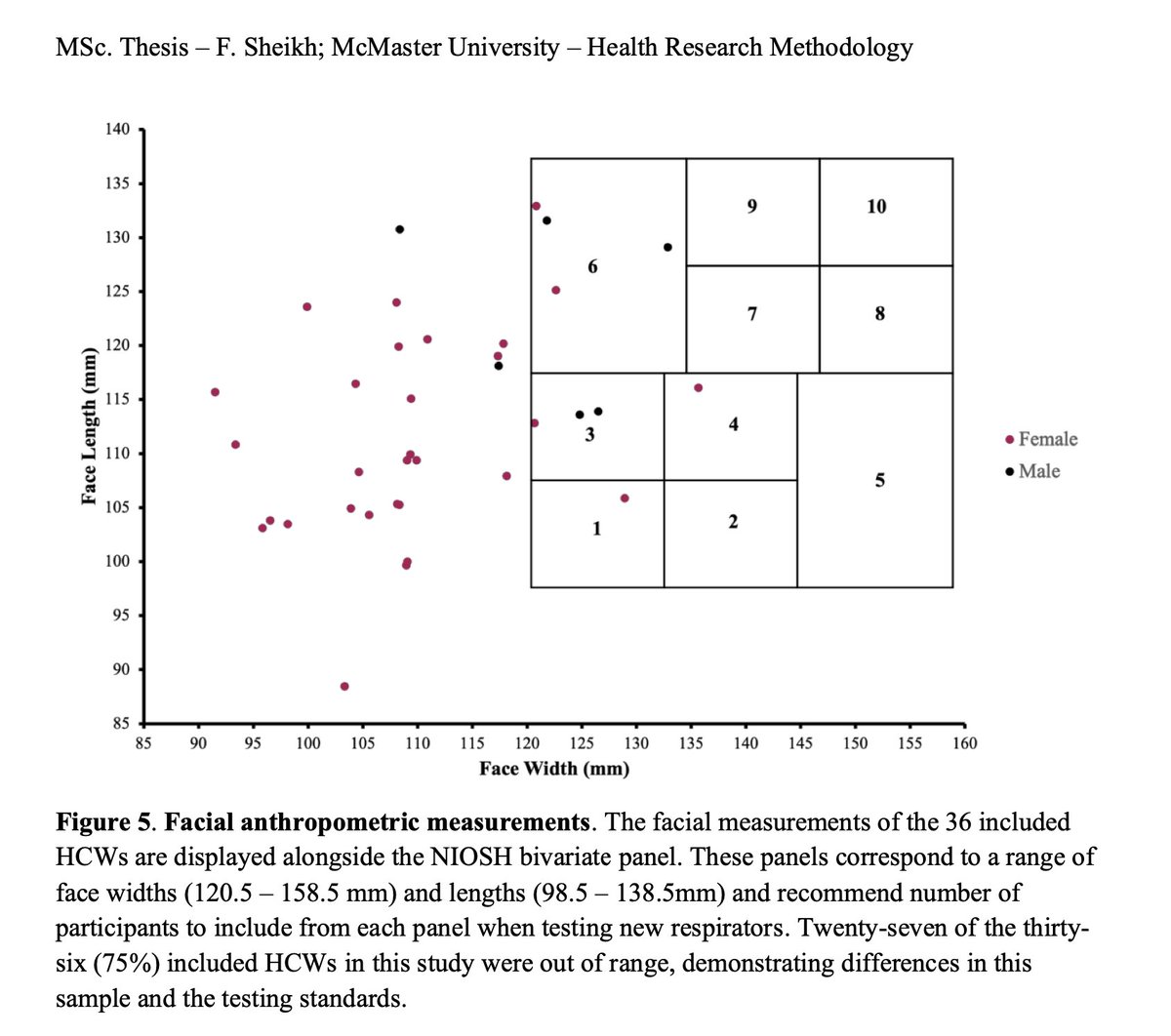
Aug 2022 MSc thesis @fatima_sheikkh:
"In Canada, women represent 82% of HCWs, but most masks and respirators have been designed based on the anthropometrics of average men in the US & Europe."
macsphere.mcmaster.ca/bitstream/1137…
/25



Aug 2022 MSc thesis @fatima_sheikkh:
"In Canada, women represent 82% of HCWs, but most masks and respirators have been designed based on the anthropometrics of average men in the US & Europe."
macsphere.mcmaster.ca/bitstream/1137…
/25



My thanks to @ghhughes for the thread.
Past bedtime for me, but I will pick up on this later,
/26
https://twitter.com/ghhughes/status/1603462358957166592?s=20&t=IiF9YWfq8sKIZjMNsaEnOA
Past bedtime for me, but I will pick up on this later,
/26
As @larmbrust shows in his video, the 3M 8210 cup edge presses tightly against the face, even through stubble. Reliable seal, but tough on skin.
It's also a stiff mask that clamps the mouth a bit, and dampens the voice, affecting HCW who speak more than industrial workers.
/27
It's also a stiff mask that clamps the mouth a bit, and dampens the voice, affecting HCW who speak more than industrial workers.
/27
@larmbrust The 3M 8210 and 8110s (for small) were approved by NIOSH in 1995.
They became entrenched in industrial workplaces which require regular fit-testing, because the pressure of the cup edge on skin helps ensure a seal on many faces.
Male faces.
/28
wwwn.cdc.gov/NIOSH-CEL/Appr…
They became entrenched in industrial workplaces which require regular fit-testing, because the pressure of the cup edge on skin helps ensure a seal on many faces.
Male faces.
/28
wwwn.cdc.gov/NIOSH-CEL/Appr…
Informative conversation between @masknerd and 3M's Nikki McCullough.
Millions of users around the world have been fit-tested to a specific 3M cup-style N95. So 3M improves things like comfort as far as they can without changing that form factor.
/29
news.3m.com/Q-A-Mask-Nerd-…
Millions of users around the world have been fit-tested to a specific 3M cup-style N95. So 3M improves things like comfort as far as they can without changing that form factor.
/29
news.3m.com/Q-A-Mask-Nerd-…
So 3M developed the radically different 9105 VFlex duckbill, which NIOSH approved in 2010.
wwwn.cdc.gov/NIOSH-CEL/Appr…
@larmbrust testing shows the difference in breathing resistance:
• 8210 cup - 100 Pa
• 1804 VFlex - 40 Pa
As low as a flat mask!
/30
armbrustusa.com/blogs/mask-rev…
wwwn.cdc.gov/NIOSH-CEL/Appr…
@larmbrust testing shows the difference in breathing resistance:
• 8210 cup - 100 Pa
• 1804 VFlex - 40 Pa
As low as a flat mask!
/30
armbrustusa.com/blogs/mask-rev…
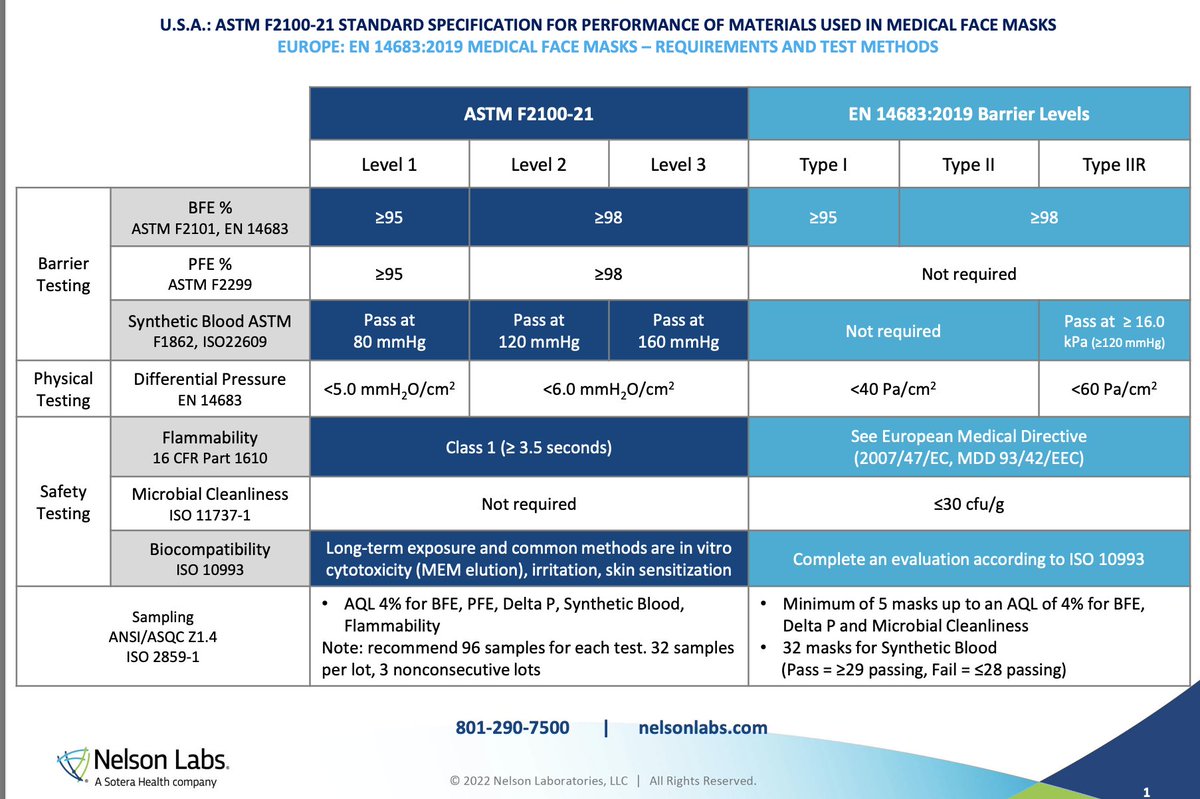
@larmbrust 3M's marketing literature claims the "Healthcare" versions of their N95 have around the same breathability of ASTM F2100 flat masks. 5 mm H2O is about 50 Pa.
I have worn both the Orpyx L3 flat masks and the 3M 1804 VFlex N95, and prefer the N95.
/31
multimedia.3m.com/mws/media/1387…
I have worn both the Orpyx L3 flat masks and the 3M 1804 VFlex N95, and prefer the N95.
/31
multimedia.3m.com/mws/media/1387…

Please bear with me on these details on 3M's N95.
I volunteer with many HCW and other professionals who do not have this reference information.
If you work for AHS, your health & safety depend on getting information directly from 3M and other reputable manufacturers.
/32
I volunteer with many HCW and other professionals who do not have this reference information.
If you work for AHS, your health & safety depend on getting information directly from 3M and other reputable manufacturers.
/32
Did you notice 3M compared breathing resistance:
• only for their Healthcare N95 (40 to 60 Pa)
• against the (Halyard) competition (100 - 120 Pa)
• but not their Unvalved N95 (100 - 125 Pa)
• eg the 8210 which AHS decided on for HCW (100 Pa)
/33
multimedia.3m.com/mws/media/1434…



• only for their Healthcare N95 (40 to 60 Pa)
• against the (Halyard) competition (100 - 120 Pa)
• but not their Unvalved N95 (100 - 125 Pa)
• eg the 8210 which AHS decided on for HCW (100 Pa)
/33
multimedia.3m.com/mws/media/1434…
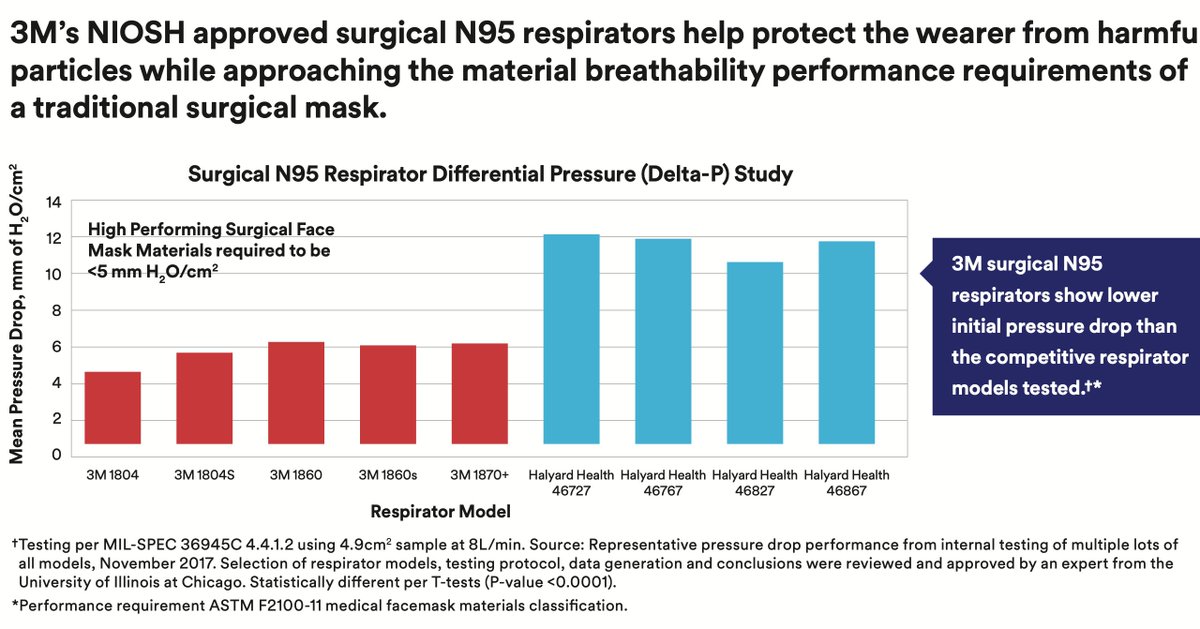



Why?
• Many workers prefer N95 with an unfiltered exhalation valve, to keep the inside of their respirators cool, and their breathing easier
• HCW cannot have that
• So 3M makes these specialized versions of popular N95 forms, with lower breathing resistance, for HCW.
/34
• Many workers prefer N95 with an unfiltered exhalation valve, to keep the inside of their respirators cool, and their breathing easier
• HCW cannot have that
• So 3M makes these specialized versions of popular N95 forms, with lower breathing resistance, for HCW.
/34
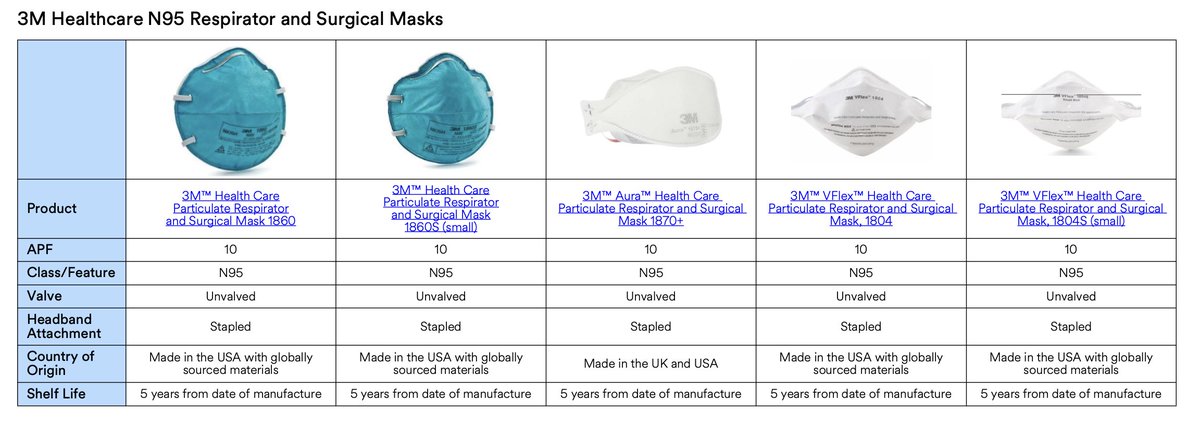
Serious problem from AHS deciding on 8210/8110s as fit-tested N95 for HCW.
• They're not fluid resistant 💥😱
• Procedural masks are fluid-resistant
• AHS defines COVID-19 as contact/droplet hazard
• HCW required to add eye protection, not face shield
albertahealthservices.ca/assets/healthi…



• They're not fluid resistant 💥😱
• Procedural masks are fluid-resistant
• AHS defines COVID-19 as contact/droplet hazard
• HCW required to add eye protection, not face shield
albertahealthservices.ca/assets/healthi…




@jvipondmd @demandsbetter @TheBreakdownAB @cricket_of_MH @PopAlberta @sandraiaz @DanielleLarivee @gilmcgowan @MikeParkerHSAA @RajBhardwajMD Sorry, the above was tweet /35 in the thread, and it raises serious concerns about HCW protection from contact hazard in the 8210/8110s AHS selected.
3M lays it out very clearly, because NIOSH approval for N95 does not include fluid resistance.
/36
multimedia.3m.com/mws/media/1839…
3M lays it out very clearly, because NIOSH approval for N95 does not include fluid resistance.
/36
multimedia.3m.com/mws/media/1839…
@jvipondmd @demandsbetter @TheBreakdownAB @cricket_of_MH @PopAlberta @sandraiaz @DanielleLarivee @gilmcgowan @MikeParkerHSAA @RajBhardwajMD Thanks @cricket_of_MH - I know it is common Practice to wear a procedural mask on top of an N95 for fluid protection, but this is unsafe and against Policy.
"Layering or double masking is not recommended in any circumstance."
albertahealthservices.ca/assets/info/pp…


"Layering or double masking is not recommended in any circumstance."
albertahealthservices.ca/assets/info/pp…
https://twitter.com/cricket_of_MH/status/1609194229481500674?s=20&t=_pfbnBAQOhjZWvOeYfjv1Q


@jvipondmd @demandsbetter @TheBreakdownAB @cricket_of_MH @PopAlberta @sandraiaz @DanielleLarivee @gilmcgowan @MikeParkerHSAA @RajBhardwajMD @JenLeeCBC @Adam_Toy @Jantafrench @politicalham @CTVdawnwalton @CAAerosolCltn @KevinHedges15 @kristatee @dewigmore @mariannelev Summer 2020: AHS abandoned fluid-resistant 3M Aura 1870 to which HCW had been fit-tested, and switched to NON fluid-resistant 3M cup 8210/8110s.
They should have provided fluid-resistant replacement, or at least required face shield with those N95.
/38

They should have provided fluid-resistant replacement, or at least required face shield with those N95.
/38
https://twitter.com/ZiadFazel/status/1608338159548633094?s=20&t=_pfbnBAQOhjZWvOeYfjv1Q

@jvipondmd @demandsbetter @TheBreakdownAB @cricket_of_MH @PopAlberta @sandraiaz @DanielleLarivee @gilmcgowan @MikeParkerHSAA @RajBhardwajMD @JenLeeCBC @Adam_Toy @Jantafrench @politicalham @CTVdawnwalton @CAAerosolCltn @KevinHedges15 @kristatee @dewigmore @mariannelev Please note: I will be making formal regulatory complaints about issues in this thread, as I did in Feb 2022 over the BYD Children's Masks that LaGrange issued to schoolchildren.
I'm using a thread to raise awareness and gather your help/feedback, but it won't end here.
/39
I'm using a thread to raise awareness and gather your help/feedback, but it won't end here.
/39
@jvipondmd @demandsbetter @TheBreakdownAB @cricket_of_MH @PopAlberta @sandraiaz @DanielleLarivee @gilmcgowan @MikeParkerHSAA @RajBhardwajMD @JenLeeCBC @Adam_Toy @Jantafrench @politicalham @CTVdawnwalton @CAAerosolCltn @KevinHedges15 @kristatee @dewigmore @mariannelev Quick recap. IMO, AHS/Orpyx deal in summer 2020 was $60m boondoggle:
• For $23m they could have joined ON & Fed Govs to get 25m Healthcare N95 per year from 3M Brockville expansion
• For $6m they could get 40m ASTM procedural masks from @CAPPEM2
/40
• For $23m they could have joined ON & Fed Govs to get 25m Healthcare N95 per year from 3M Brockville expansion
• For $6m they could get 40m ASTM procedural masks from @CAPPEM2
/40
https://twitter.com/BarryHunt008/status/1608803603430662146?s=20&t=_pfbnBAQOhjZWvOeYfjv1Q
@jvipondmd @demandsbetter @TheBreakdownAB @cricket_of_MH @PopAlberta @sandraiaz @DanielleLarivee @gilmcgowan @MikeParkerHSAA @RajBhardwajMD @JenLeeCBC @Adam_Toy @Jantafrench @politicalham @CTVdawnwalton @CAAerosolCltn @KevinHedges15 @kristatee @dewigmore @mariannelev @CAPPEM2 Since Fall 2020, IMO, AHS *continues to deprive* HCW of acceptable fit-tested respirators:
• Overcommitted to Shanghai Dasheng instead of 🇨🇦
• Still rejecting @CAPPEM2 offers of @GovCanHealth approved respirators
• Dec 2021: Bought BYD KN95 trash
/42
• Overcommitted to Shanghai Dasheng instead of 🇨🇦
• Still rejecting @CAPPEM2 offers of @GovCanHealth approved respirators
• Dec 2021: Bought BYD KN95 trash
/42
https://twitter.com/BarryHunt008/status/1608806080095846400?s=20&t=_pfbnBAQOhjZWvOeYfjv1Q
I believe AHS is buying this BYD KN95 trash from MHCare Medical, the Edmonton distributor that brought us Vanch masks in 2020.
@BYDCompany authorized 🇨🇦distributor told me in Jan 2022 - when I reported them to @GovCanHealth - they were leaving the flat mask & KN95 business.
/43
@BYDCompany authorized 🇨🇦distributor told me in Jan 2022 - when I reported them to @GovCanHealth - they were leaving the flat mask & KN95 business.
/43

MHCare Medical is the healthcare venture from Mraiche Holding Corporation, who brought us the Vanch mask fiasco in April 2020.
@kim_siever covers the background very well here.
Instead of dumping MHCare Medical, AHS has instead gotten MUCH deeper.
/44
kim-siever.medium.com/what-you-may-n…
@kim_siever covers the background very well here.
Instead of dumping MHCare Medical, AHS has instead gotten MUCH deeper.
/44
kim-siever.medium.com/what-you-may-n…
@kim_siever Thanks, Kim. I collected boxes & QC certificates - between us we have 4 different manufacturers.
I believe Vānch is just the PPE brand of stationery supplier Beifa Group, who bought whatever mani-pedi flat masks they could find, and repackaged them.
/45




I believe Vānch is just the PPE brand of stationery supplier Beifa Group, who bought whatever mani-pedi flat masks they could find, and repackaged them.
/45
https://twitter.com/kim_siever/status/1609298467280805889?s=20&t=_pfbnBAQOhjZWvOeYfjv1Q




@kim_siever On 20 Oct 2022, Supply Chain Advancement Network in Health held Dialogue Forum I in Edmonton, hosted & sponsored by Alberta Innovates, co-sponsored by Shoppers Drug Mart & GS1 Canada.
Chief Program Officer for AHS CPSM gave an interesting Keynote.
/46
scanhealth.ca/news-events/di…



Chief Program Officer for AHS CPSM gave an interesting Keynote.
/46
scanhealth.ca/news-events/di…




@kim_siever @CAPPEM2 @laurby @TheBreakdownAB @Jantafrench @Adam_Toy @JenLeeCBC @jvipondmd @CTVdawnwalton @morganrblack ~1-hr mark, Prasad complains about AHS comms making him "get the right story out" about the Vanch procedural masks at #COVID19AB theatre, be "grilled by a bunch of reporters about the quality of the masks", only to have people say "I know better".
/47
/47
20 April 2020 #COVID19AB Theatre at which Prasad presented. Total 55 min, most taken by other topics like NS mass shooting, oil & gas, Cargill outbreak, and CMOH update.
The few "1 question/1 followup" he got was no grilling.
/48
The few "1 question/1 followup" he got was no grilling.
/48
I'm one of the critics who knew better:
• Chinese YY/T 0969 standard is NOT fluid resistant and does not look below 3 µm filtration
• All ASTM levels filter at 0.1µm >95%, and are fluid resistant at least 80 mmHg
• I asked to see the test results
/49


• Chinese YY/T 0969 standard is NOT fluid resistant and does not look below 3 µm filtration
• All ASTM levels filter at 0.1µm >95%, and are fluid resistant at least 80 mmHg
• I asked to see the test results
/49
https://twitter.com/ZiadFazel/status/1253068076527915008?s=20&t=VpTAeF88x7iD1rTynvpPsA

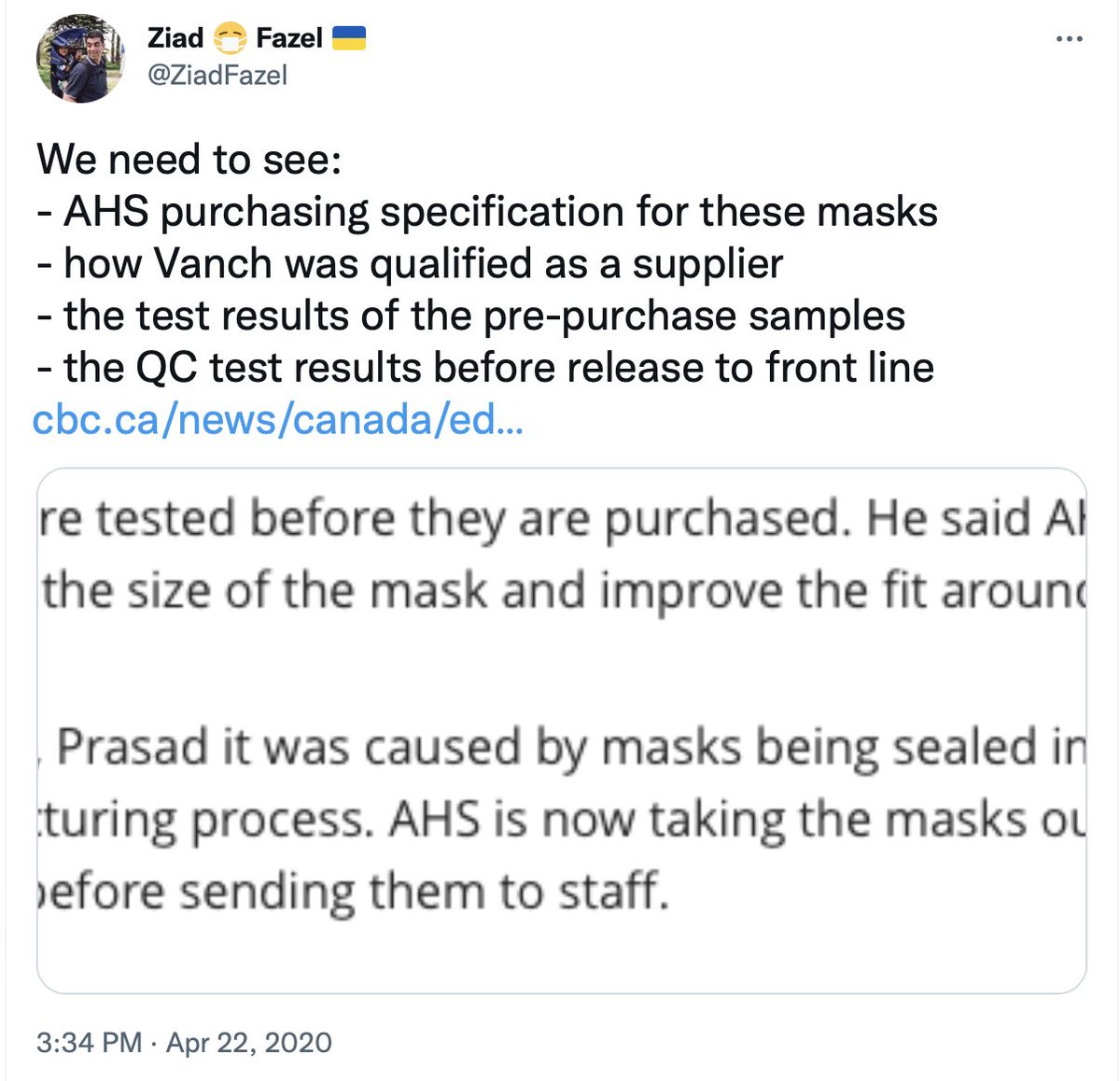
Around 27 April 2020, AHS put out this deceptive spin:
• impossible claims about the masks tested and meeting mutually exclusive standards
• but not releasing those test results
• setting up channels for staff to complain internally, not to media
/50
albertahealthservices.ca/assets/info/pp…
• impossible claims about the masks tested and meeting mutually exclusive standards
• but not releasing those test results
• setting up channels for staff to complain internally, not to media
/50
albertahealthservices.ca/assets/info/pp…

~1:01 of Keynote, Prasad says AHS decided not to respond further to concerns raised about the Vanch masks.
Not even the 150+ doctors who signed this specific, accurate letter citing their sources.
/c @laurby @John1MD @Arfarfarf8 @FionaMattatall
/51
calgarysun.com/news/local-new…
Not even the 150+ doctors who signed this specific, accurate letter citing their sources.
/c @laurby @John1MD @Arfarfarf8 @FionaMattatall
/51
calgarysun.com/news/local-new…
@laurby @John1MD @Arfarfarf8 @FionaMattatall AFAIK, here are 17 Mar 2020 test results AHS kept from you.
• true manufacturer Anhui calls them YMJ1
• they meet EN14683 Type II (not ASTM, not fluid resistant)
• YMJ2 is kids mask; YMJ3 meets Type IIR (fluid resistant) but is different mask
/52
autotecperlemergenza.com/ol/download/99…



• true manufacturer Anhui calls them YMJ1
• they meet EN14683 Type II (not ASTM, not fluid resistant)
• YMJ2 is kids mask; YMJ3 meets Type IIR (fluid resistant) but is different mask
/52
autotecperlemergenza.com/ol/download/99…




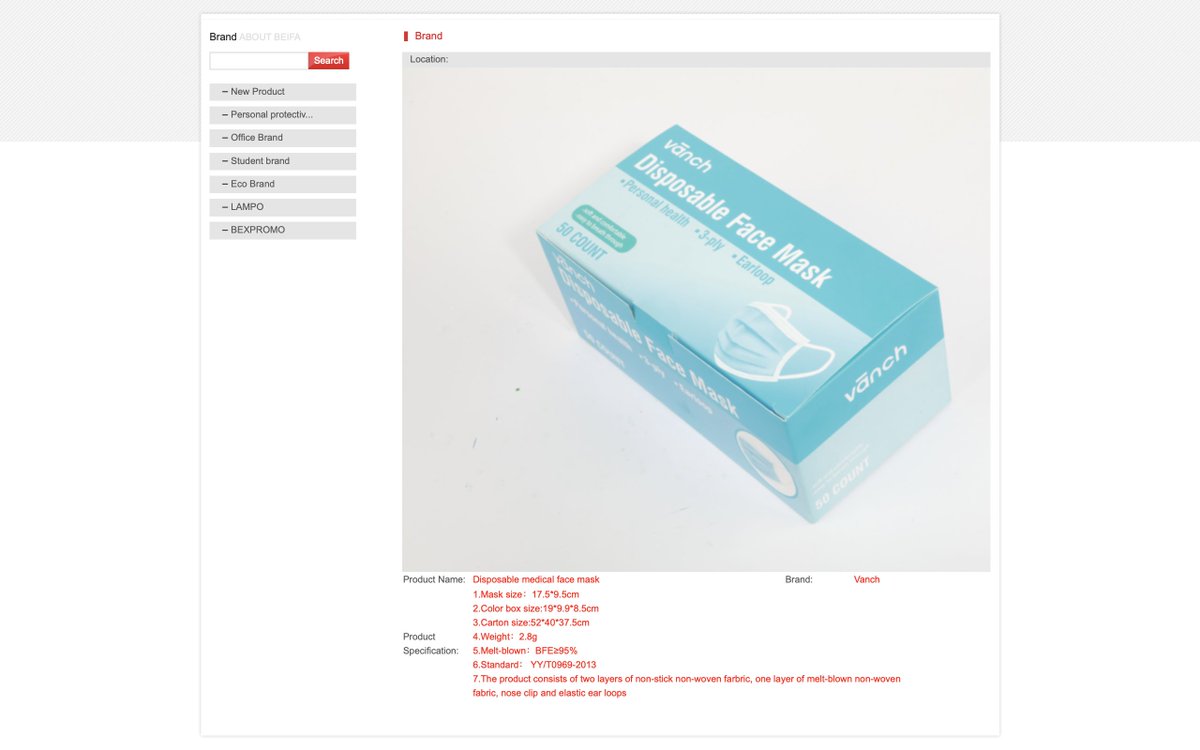
@laurby @John1MD @Arfarfarf8 @FionaMattatall @TheBreakdownAB @Jantafrench @CAPPEM2 @RajBhardwajMD @kristatee @Adam_Toy @dupuisj @CAAerosolCltn @dewigmore @sameo416 .@kim_siever and I count 4 different YY/T 0969 contract manufacturers under Vānch brand for Beifa distributor in China. They put Beifa's KZ001 code on the QC Cert.
Beifa may have sourced from MANY more to meet the orders.
/53
beifa.com/en/productsd.p…


Beifa may have sourced from MANY more to meet the orders.
/53
beifa.com/en/productsd.p…
https://twitter.com/ZiadFazel/status/1609310561384411137?s=20&t=lNOUyi8W2FtE4GmOiXBxRQ

@laurby @John1MD @Arfarfarf8 @FionaMattatall @TheBreakdownAB @Jantafrench @CAPPEM2 @RajBhardwajMD @kristatee @Adam_Toy @dupuisj @CAAerosolCltn @dewigmore @sameo416 @kim_siever "PPE Quality Group" to "clinically “field test” incoming products prior to sending them into circulation".
ie. Guinea pig for whatever Beifa & MH Care Medical can get, under AHS's underlying deception to staff that the Vānch masks meet ASTM Level 1.
/54
albertahealthservices.ca/assets/info/pp…
ie. Guinea pig for whatever Beifa & MH Care Medical can get, under AHS's underlying deception to staff that the Vānch masks meet ASTM Level 1.
/54
albertahealthservices.ca/assets/info/pp…

@John1MD @Arfarfarf8 @FionaMattatall @TheBreakdownAB @Jantafrench @CAPPEM2 @RajBhardwajMD @kristatee @Adam_Toy @dupuisj @CAAerosolCltn @dewigmore @sameo416 @kim_siever @gilmcgowan @sandraiaz @MikeParkerHSAA @HSAAlbertaEMS @DanielleLarivee @UnitedNurses @AB_MD_WarRoom The manufacturer Anhui's own documentation couldn't be more clear about which international standards they meet.
• YMJ1 flat mask - EN 14683 Type II - NOT fluid resistant
• YMJ3 3D mask - Type IIR - fluid resistant (120 mm Hg)
/55
autotecperlemergenza.com/ol/download/99…
• YMJ1 flat mask - EN 14683 Type II - NOT fluid resistant
• YMJ3 3D mask - Type IIR - fluid resistant (120 mm Hg)
/55
autotecperlemergenza.com/ol/download/99…

When repeatedly challenged for what standard the PPE meets, and for proof it meets that, the ethical employer provides manufacturer documentation, and the March 2020 independent lab test results it claims to already have.
@AHS_media deceived Albertans with spin instead.
/56
@AHS_media deceived Albertans with spin instead.
/56
So let's recap again. In this thread, tweets:
• 1-7 Orpyx procedural masks
• 8-40 fit-tested respirators - URGENT concern over fluid resistance
• 42-56 history of deception by @AHS_media with Vanch, which destroyed their credibility to handle subsequent problems with BYD
/57
• 1-7 Orpyx procedural masks
• 8-40 fit-tested respirators - URGENT concern over fluid resistance
• 42-56 history of deception by @AHS_media with Vanch, which destroyed their credibility to handle subsequent problems with BYD
/57
@AHS_media Thank you @TheBreakdownAB for having me on the show Sunday to "Show & Tell". I started ~41 min mark and ended 45 mins later.
I am blowing the whistle on AHS CPSM and WHS, and discovering more problems as I double-check.
Many serious questions.
/58
I am blowing the whistle on AHS CPSM and WHS, and discovering more problems as I double-check.
Many serious questions.
/58
@AHS_media @TheBreakdownAB On Tue 6 Dec 2022, my parents tested positive for COVID-19 at their Carewest LTC, by rapid tests I conducted (later PCR-confirmed).
While I have always been concerned about AHS IPC, this made those policies and the PPE on the AHS isolation cart my business to know.
/59
While I have always been concerned about AHS IPC, this made those policies and the PPE on the AHS isolation cart my business to know.
/59

Why did I rapid-test my parents? Because Carewest policy before they will authorize antiviral therapy - confirmed by physician & RN in charge - is to wait for PCR results.
Which is 2 days, or Wed at earliest for Mum. Dad was not swabbed until he was symptomatic Tuesday.
/60
Which is 2 days, or Wed at earliest for Mum. Dad was not swabbed until he was symptomatic Tuesday.
/60
I looked up the AHS policy for Paxlovid & Remdesivir after my parents' doc called me Monday. Although he had told me we had to wait until the PCR result, AHS policy since Sep 2022 accepted a positive Rapid Antigen Test too. Every day is crucial.
/61
albertahealthservices.ca/topics/Page177…
/61
albertahealthservices.ca/topics/Page177…
Busy HCW have not kept up with the changing availability & eligibility of these antiviral therapeutics, but they have been badly underused all year.
@JenLeeCBC April:
cbc.ca/news/canada/ca…
@CBCQueensPark September:
cbc.ca/news/health/co…
/62
@JenLeeCBC April:
cbc.ca/news/canada/ca…
@CBCQueensPark September:
cbc.ca/news/health/co…
/62
@JenLeeCBC @CBCQueensPark I criticize other parts of AHS in this thread, but I want to compliment everyone at COVID-19 Outpatient Treatment Program.
Every contact I had with you - whether for oral Paxlovid or intravenous Remdesivir, incoming or outgoing call - was awesome.
albertahealthservices.ca/topics/Page177…
/63
Every contact I had with you - whether for oral Paxlovid or intravenous Remdesivir, incoming or outgoing call - was awesome.
albertahealthservices.ca/topics/Page177…
/63
Back to the topic of this thread:
Questionable decisions by AHS on Masks & Respirators.
In December 2021, AHS deployed massive amounts of BYD earloop KN95 for staff & visitors.
Staff HATED them. The earloops were too tight and said "like breathing through a maxipad."
/64

Questionable decisions by AHS on Masks & Respirators.
In December 2021, AHS deployed massive amounts of BYD earloop KN95 for staff & visitors.
Staff HATED them. The earloops were too tight and said "like breathing through a maxipad."
/64


Not until February before AHS Exec Dir of Workplace Health & Safety addressed them in "PPE Question of the Week."
• staff in congregate living facilities, not acute care
• can provide higher protection than procedure mask
• 2 metre or AGMP for N95
/65
• staff in congregate living facilities, not acute care
• can provide higher protection than procedure mask
• 2 metre or AGMP for N95
/65
According to the WH&S executive's video, staff in congregate or continuing care aren't supposed to wear a KN95 around COVID-19 patients?
That is NOT what the procedures say. Let's start here
albertahealthservices.ca/assets/healthi…
Modified Respiratory Precautions for COVID-19, right?
/66

That is NOT what the procedures say. Let's start here
albertahealthservices.ca/assets/healthi…
Modified Respiratory Precautions for COVID-19, right?
/66


Contrary to Resource Manuals, there's no Information Sheet for Modified Respiratory Precautions.
albertahealthservices.ca/ipc/Page6854.a…
There are only posters effective 14 Feb 2022
albertahealthservices.ca/assets/healthi…
Or this mod of contact/droplet Don/Doff on 17 Feb 2022
albertahealthservices.ca/assets/healthi…
/67



albertahealthservices.ca/ipc/Page6854.a…
There are only posters effective 14 Feb 2022
albertahealthservices.ca/assets/healthi…
Or this mod of contact/droplet Don/Doff on 17 Feb 2022
albertahealthservices.ca/assets/healthi…
/67




So on 3 Feb 2022, AHS Exec Dir of Workplace Health & Safety makes a video saying staff should not wear the KN95 instead of a fit-tested N95.
But the procedures on 14 & 17 Feb for staff in congregate or continuing care say they can wear N95 or KN95 or procedural mask
/68



But the procedures on 14 & 17 Feb for staff in congregate or continuing care say they can wear N95 or KN95 or procedural mask
/68




You may say "Ziad, be forgiving. Paperwork mistakes under pressure."
I respond:
• Why is AHS distributing KN95 to ANYONE in December 2021?! And still doing so TO THIS DAY?
• Aren't the lives of nurses & patients in congregate care just as valuable as those in acute care?!
/69
I respond:
• Why is AHS distributing KN95 to ANYONE in December 2021?! And still doing so TO THIS DAY?
• Aren't the lives of nurses & patients in congregate care just as valuable as those in acute care?!
/69
You can see this double-talk & double-standard in the PPE principles for facility screeners.
AHS claims N95 or KN95 or medical mask are all considered safe practice for continuous masking, but KN95 masks are not used at acute care sites.
RED FLAG.
/70
albertahealthservices.ca/assets/healthi…
AHS claims N95 or KN95 or medical mask are all considered safe practice for continuous masking, but KN95 masks are not used at acute care sites.
RED FLAG.
/70
albertahealthservices.ca/assets/healthi…
"Note: Provincial OHS legislation does not include non-fit tested respirators (eg KN95) in the legislative definition of a respirator. Therefore, in Alberta, KN95s are called masks rather than respirators."
AHS calls them KN95 Respirators anyway
/71
albertahealthservices.ca/assets/info/pp…
AHS calls them KN95 Respirators anyway
/71
albertahealthservices.ca/assets/info/pp…
"Do not use an N95 respirator if fit testing is not current...If fit testing is NOT available or current then don a seal-checked KN95 mask or well-fitting procedure/surgical mask."
Staff can't wear seal-checked N95. I've asked.
KN95.
Anyone seal-checked a procedure mask?
/72
Staff can't wear seal-checked N95. I've asked.
KN95.
Anyone seal-checked a procedure mask?
/72
Now, the most serious issues with BYD KN95.
Effective 6 July 2021, US FDA revoked Emergency Use Authorization for both BYD KN95:
• DG3101 earloop
• DN1102 headband
US Hospitals use N95s for COVID-19; they don't pretend procedural masks are OK.
/73
fda.gov/medical-device…
Effective 6 July 2021, US FDA revoked Emergency Use Authorization for both BYD KN95:
• DG3101 earloop
• DN1102 headband
US Hospitals use N95s for COVID-19; they don't pretend procedural masks are OK.
/73
fda.gov/medical-device…

Since 2020, as US FDA revoked EUA for KN95:
• performance reasons at any time
• July 2021 when N95 supply caught up to demand
Health Canada required manufacturers & importers to apply for an Interim Order to be considered respirators in Canada.
/74
canada.ca/en/health-cana…
• performance reasons at any time
• July 2021 when N95 supply caught up to demand
Health Canada required manufacturers & importers to apply for an Interim Order to be considered respirators in Canada.
/74
canada.ca/en/health-cana…

Without approval under an Interim Order from Health Canada:
• either as a medical-grade mask under ASTM F2100 or EN 14683
• or as a respirator under CSA Z94 or interim🇨🇦 guidance
The BYD KN95 could not be medical ANYTHING.
Only "face masks" like a common dust mask.
/75
• either as a medical-grade mask under ASTM F2100 or EN 14683
• or as a respirator under CSA Z94 or interim🇨🇦 guidance
The BYD KN95 could not be medical ANYTHING.
Only "face masks" like a common dust mask.
/75
After FDA revocation, neither BYD, nor any importer or distributor representing them, obtained Health Canada approval as a medical device.
So why is AHS distributing them to visitors at all sites, and to staff at congregate care as N95-equivalent PPE?!
canada.ca/en/health-cana…
So why is AHS distributing them to visitors at all sites, and to staff at congregate care as N95-equivalent PPE?!
canada.ca/en/health-cana…
@TheBreakdownAB @CAAerosolCltn @PopAlberta @DanielleLarivee @sandraiaz @CAPPEM2 @gilmcgowan @MikeParkerHSAA @OhCasavant @JenLeeCBC I went after BYD hard in Jan 2022, because they would not release their test results explaining why BYD Children's Masks had a 4-hr exposure limit.
They told me they did not sell the masks to GoA.
BYD Canada had dumped mask & KN95, leaving only N95.
/77
They told me they did not sell the masks to GoA.
BYD Canada had dumped mask & KN95, leaving only N95.
/77
https://twitter.com/ZiadFazel/status/1489078202890801154?s=20&t=_Q1ocQUe2g7U91WvLzkOyg
You can see this on BYD Canada's website. The only PPE they've been selling for the last year has been their N95.
bydcare.ca/store
Their adult surgical mask is out of stock. They have removed all traces of the children's mask from their catalog.
bydcare.ca/catalog-1
bydcare.ca/store
Their adult surgical mask is out of stock. They have removed all traces of the children's mask from their catalog.
bydcare.ca/catalog-1

So who would have picked up BYD Canada's liquidation of defective children's masks & unauthorized KN95 "respirators" at the end of 2021, and resold them to GoA?
Maybe MHCare Medical, the same people behind the Vanch masks. That AHS falsely claimed were ASTM Level 1.
/79

Maybe MHCare Medical, the same people behind the Vanch masks. That AHS falsely claimed were ASTM Level 1.
/79


Even if we ignore that AHS is providing staff BYD KN95 as PPE without Health Canada or FDA approval, they are still unbearable masks.
NIOSH tested them in April 2020.
• 223 Pa breathing resistance
• nearly 4x the maximum for a procedural mask
cdc.gov/niosh/npptl/re…
/80



NIOSH tested them in April 2020.
• 223 Pa breathing resistance
• nearly 4x the maximum for a procedural mask
cdc.gov/niosh/npptl/re…
/80



No wonder AHS staff say BYD KN95 are like breathing through a maxi pad.
Like breathing through 4+ procedural masks stacked on top of each other.
>100 KN95 passed filtration test in @larmbrust database, BYD KN95 had 5th worst breathing resistance.
/81
armbrustusa.com/pages/mask-tes…
Like breathing through 4+ procedural masks stacked on top of each other.
>100 KN95 passed filtration test in @larmbrust database, BYD KN95 had 5th worst breathing resistance.
/81
armbrustusa.com/pages/mask-tes…
You might think AHS got a really good deal on these KN95, and they are much cheaper than a flat procedural mask or an N95.
Wrong.
• BYD earloop KN95 $1 each
• 3M 8210 cup N95 $0.70
• 3M medical-grade Aura $0.96
• Primed procedural mask $0.61
albertahealthservices.ca/topics/Page169…
/82


Wrong.
• BYD earloop KN95 $1 each
• 3M 8210 cup N95 $0.70
• 3M medical-grade Aura $0.96
• Primed procedural mask $0.61
albertahealthservices.ca/topics/Page169…
/82



So I have to ask AHS:
• what was the point of ordering KN95 when the need is N95?
• how is a non-fit-tested KN95 better than a non-fit-tested N95?
• let's see your due diligence
IMHO, it looks more like enriching the supplier at the expense of staff and visitor safety.
/83
• what was the point of ordering KN95 when the need is N95?
• how is a non-fit-tested KN95 better than a non-fit-tested N95?
• let's see your due diligence
IMHO, it looks more like enriching the supplier at the expense of staff and visitor safety.
/83
• • •
Missing some Tweet in this thread? You can try to
force a refresh